Neutralisation of 30 gm of a mixture of acetic acid and phenol solutions required 100 ml of 2M sodium hydroxide solution. When the same mixture was treated with bromine water, 33.1 gm of precipitate was formed. Determine the mass percentage of acetic acid and phenol in the given solution.
Text Solution
i. 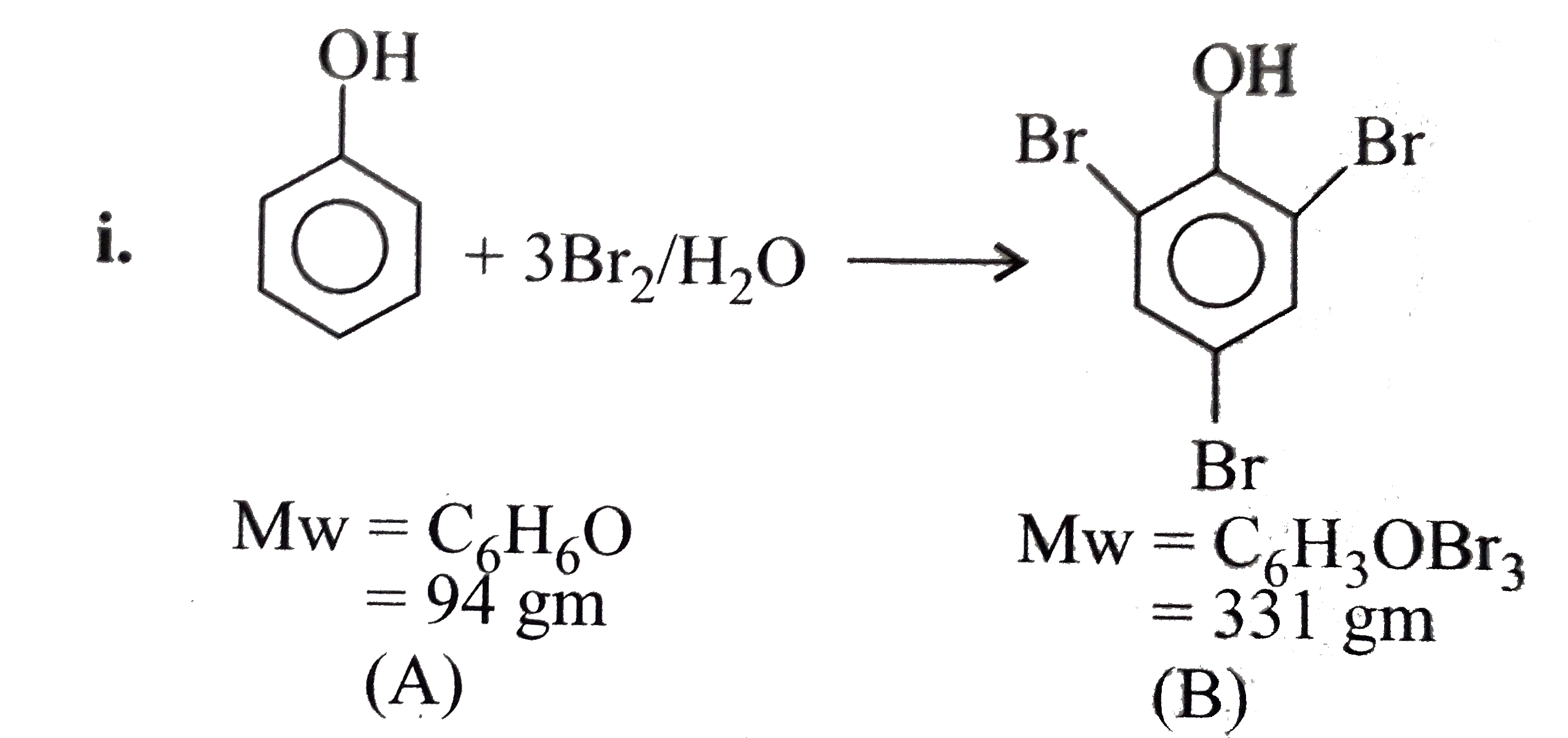
331 gm of (B) is obtained form 94 gm of (A).
33.1 gm (B) is obtained form =94331×33.1
=9.4 gm of phenol
Weight of phenol =9.4 gm
=9.49.4=0.1 mol
ii. NaOH will react with both CH3COOH and phenol. Total molar equivalent of NaOH =100×2
=200 mEq
=2001000=0.2 Eq. .of NaOH
=0.2 mol of NaOH
Acid + Phenol =0.2 mol
Acid + 0.1 mol =0.2 mol
∴ Acid =0.2−0.1=0.1 mol
=0.1 Eq.
1 Eq. of CH3COOH=60 gm
0.1 Eq. of CH3COOH=6 gm
Weight of acid = 6 gm
Weight of phenol =9.4 gm
Mass percentage of acid =630×100=20%
Mass percentage of phenol =9.430×100=31.3%
|
Similar Questions
Knowledge Check
Which one of the following solutions of sulphuric acid will exactly neutralise 25 ml of 0.2 M sodium hydroxide solution ?
A12.5ml of 0.1M solutionB25ml of 0.1 M solutionC25ml of 0.2 M solutionD50 ml of 0.2 M solution100mL of 0.3N-Acetic acid solution is mixed with same volume of 0.2N sodium hydroxide solution. Ionisation constant of acetic acid is 2×10−5 the pH of the mixture is
A10B7C5D9If 15 gm of a solute in 100 gm of water makes a solution that freezes at −1.0∘C ,then 30 gm of the same solution the freezes at
A−0.5∘CB−2.0∘CC0∘CD2.0∘C
Name the salt formed when hydrochloric acid neutralises sodium hydroxide solution.
View SolutionA 0.535 gm mixture of ethanol and acetaldehyde when heated with Fehling's solution gave 1.2 gm of a red precipitate. What is the percentage of acetaldethyde in the mixture ?
View SolutionA 0.535 gm mixture of ethanol and acetaldehyde when heated with Fehling's solution gave 1.2 gm of a red precipitate. What is the percentage of acetaldethyde in the mixture ?
View SolutionIn a 40 litres mixture acetic acid and sodium acetate are in the ratio 3:1,find the amount of sodium acetate solution to be added to make the ratio 2:3.
View Solution12 mL of 0.2 N sulphuric acid is neutralised with 15 mL of sodium hydroxide solution on titration. Calculate the normality of sodium hydroxide solution.
View Solution

