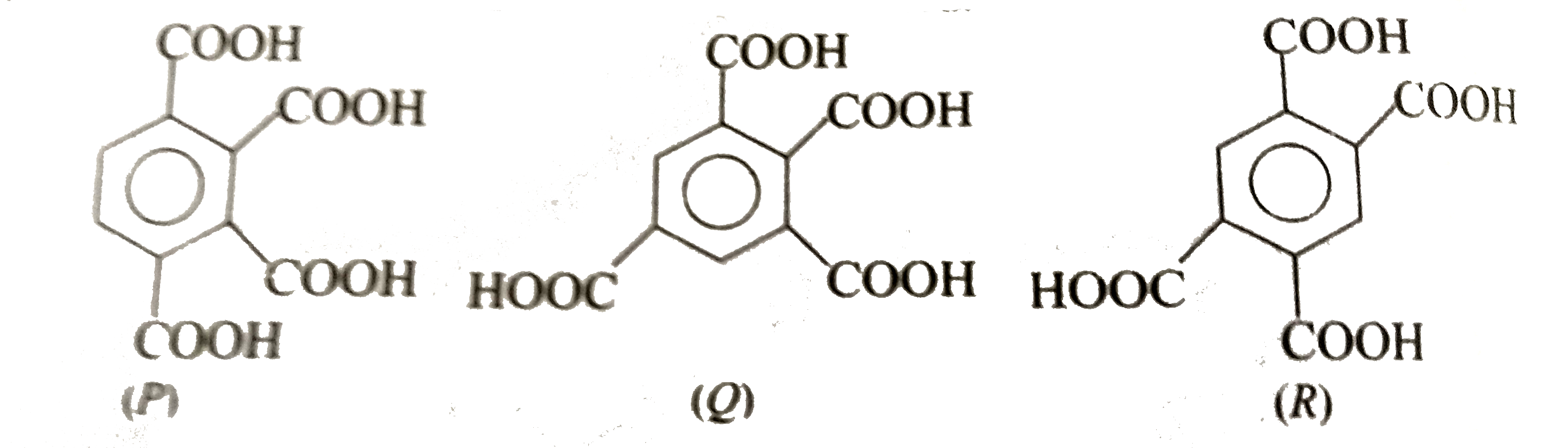
Three isomeric tetracarboxylic acids (I),(II), and (III) are given.
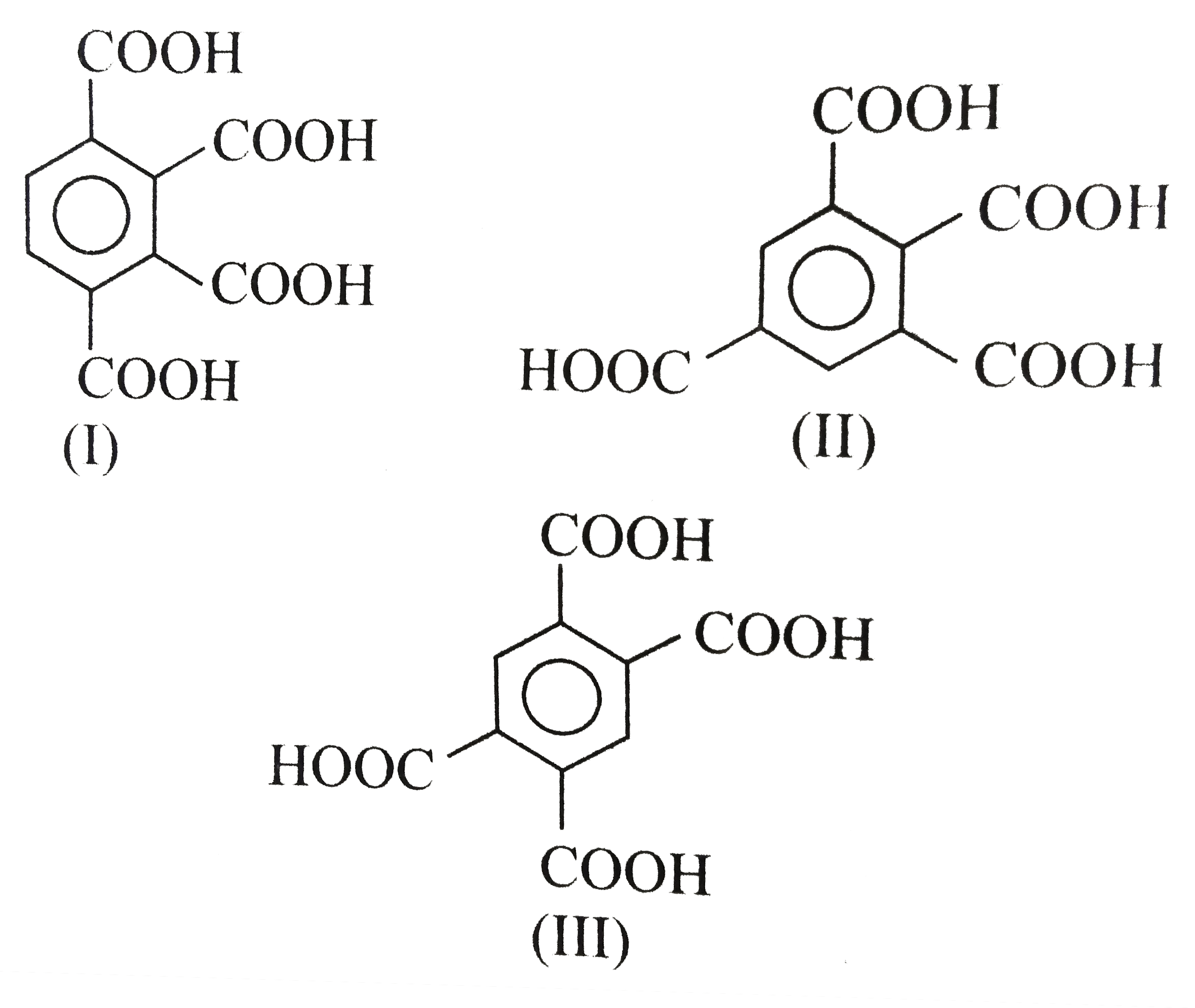
They can be distinguised by anhydride formation by treating with one equivalent and two equivalents of SOCl2.
Which tetracarboxylic acid on reaction with one equivalent of SOCl2 gives two isomeric monoanhydrides ?
(I)
(II)
(III)
All
The correct Answer is:A
(a) 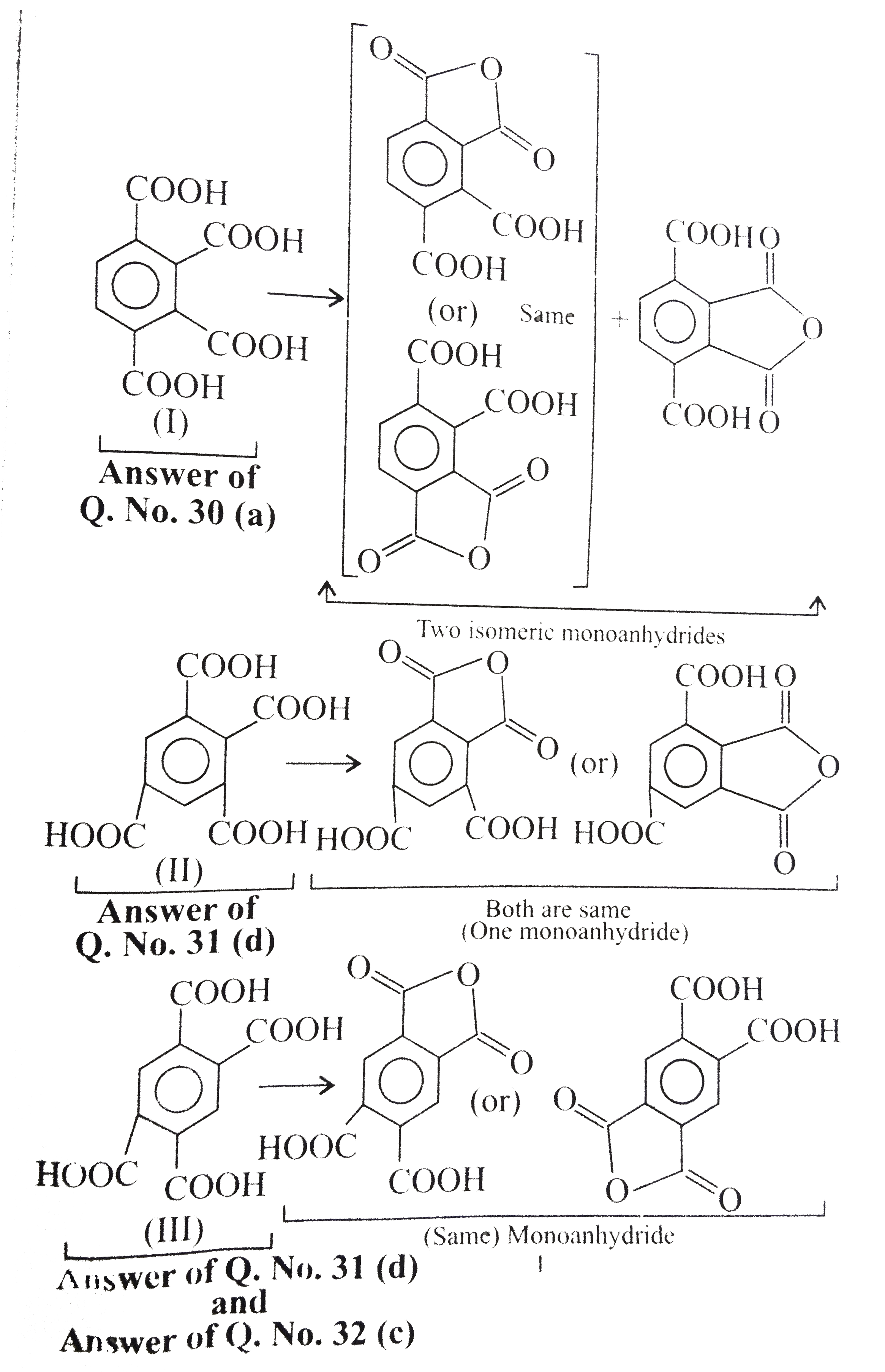 .
.
Similar Questions
Three isomeric tetracarboxylic acids (I),(II), and (III) are given.
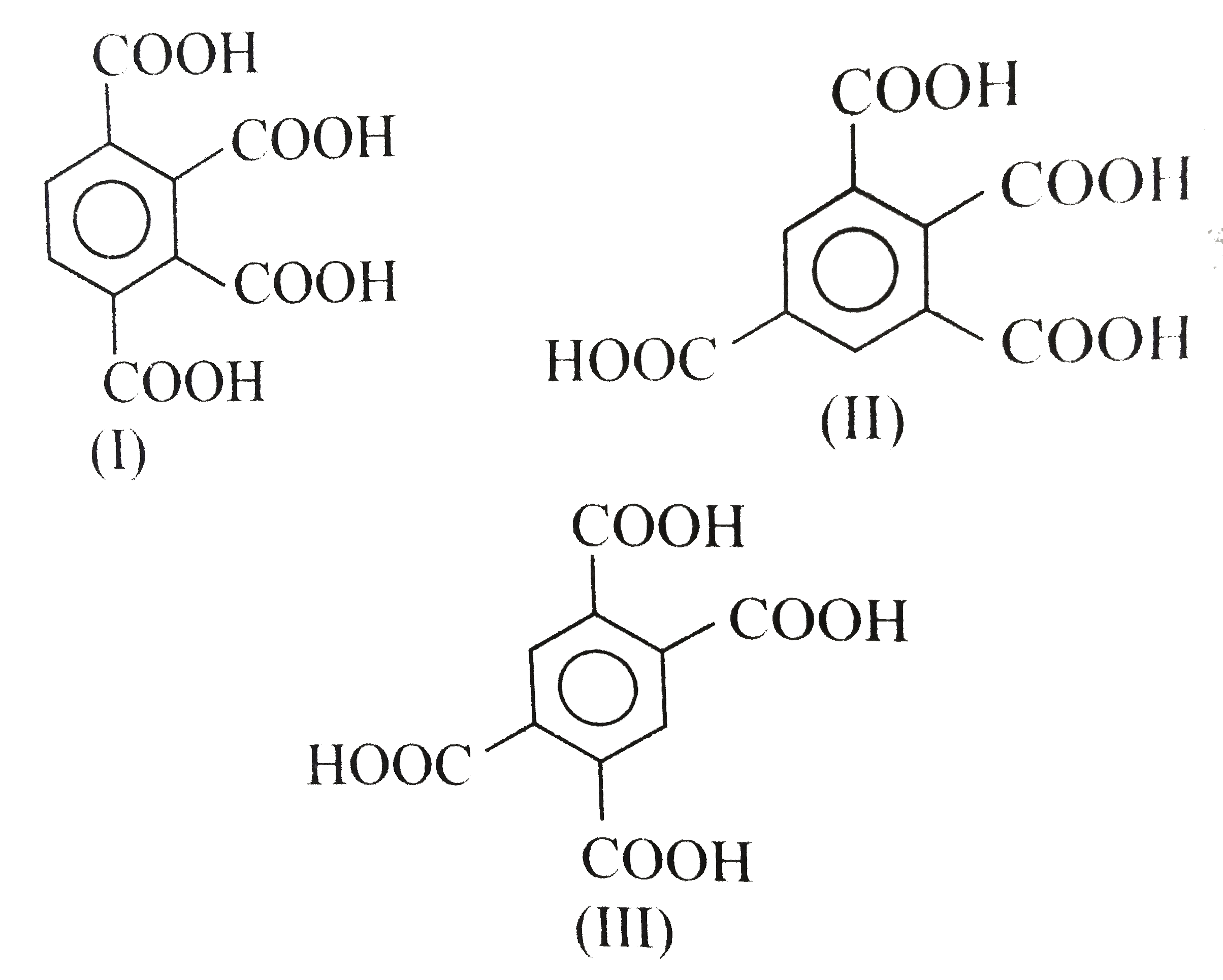
They can be distinguised by anhydride formation by treating with one equivalent and two equivalents of SOCl2.
Which tetracarboxylic acid on reaction with one equivalent of SOCl2 gives only one monoaanhydride ?
View SolutionThree isomeric tetracarboxylic acids (I),(II), and (III) are given.
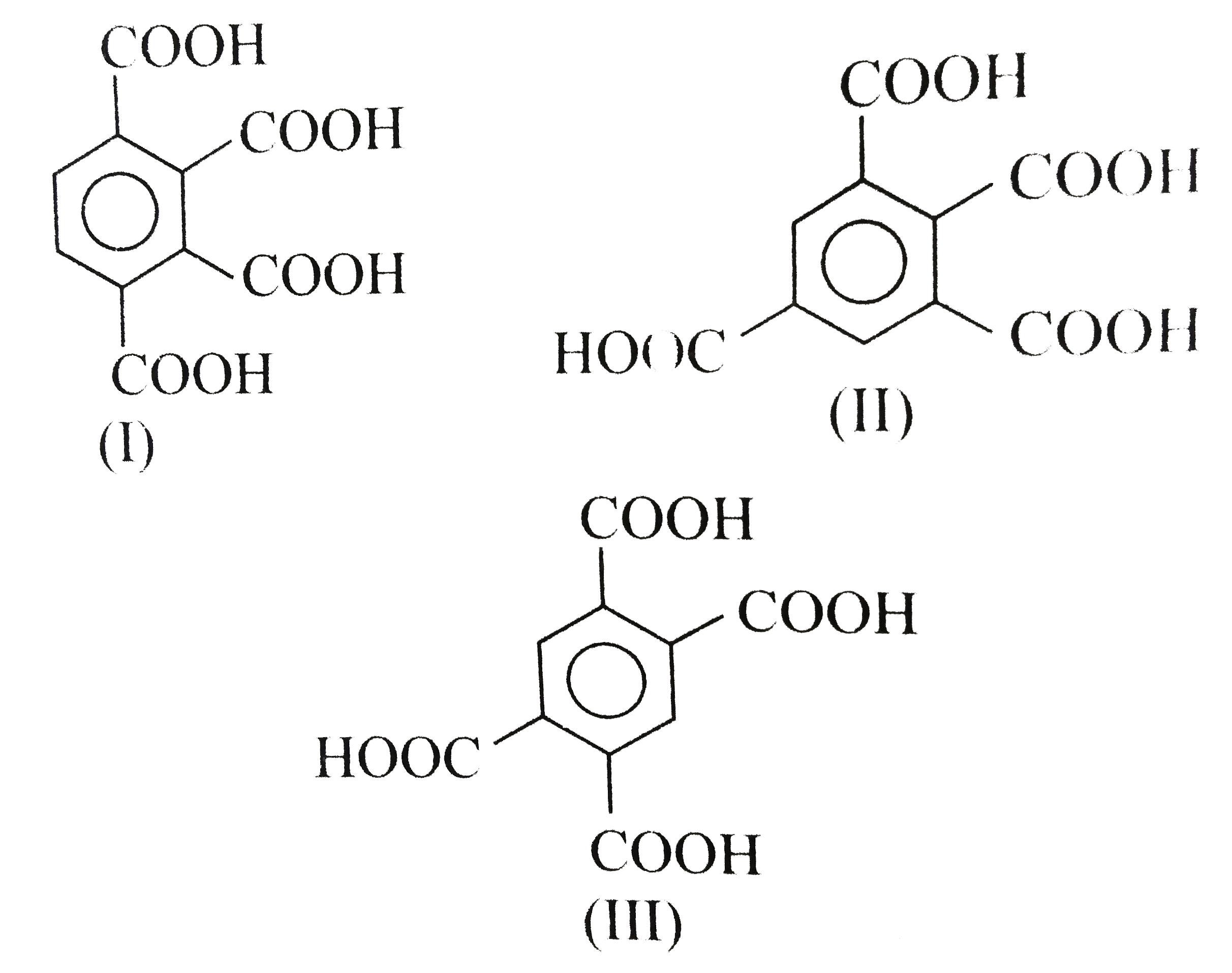
They can be distinguised by anhydride formation by treating with one equivalent and two equivalents of SOCl2.
Which tetracarboxylic acid on reaction with second equivalent of SOCl2 to the monoanhydride of (I),(II), and (III) gives a dianhydride ?
View SolutionThe reaction of one equivalent of HBr with
CH2=CH−C≡CH gives
View SolutionThere are three isomeric tetracarboxylic acid.
Which of these form two isomeric monoanhydrides ?
View SolutionThe reaction of one equivalent of HBr with CH2=CH−CH2−C≡CH gives :
View SolutionThe coordination compound of which one of the following compositions will produce two equivalents of AgCl on reaction with aqueous silver nitrate solution ?
View SolutionThe co-ordination compound of which one of the following compositions will produce two equivalents of AgCl on reaction with aqueous silver nitrate solution
View SolutionAn n-p-n transistor can be considered to be equivalent to two diodes, connected. Which of the following figures is the correct one ?
View SolutionAmides undergo hydrolysis to yield carboxylic acid plus amine on heating in either aqueous acid or aqueous base. The conditions required for amide hydrolysis are more severe than those required for the hydrolysis of esters, anhydrides or acid chlorides, but the mechanism is similar (nucleophilic acyl substitution). Nucleophilic acyl substitutions involve a tetrahedral intermediate, hence these are quite different from alkyl substitution
(RCH2BrNaCN−−−→RCH2CN)
which involves a pentavalent intermediate or transition state. One of the important reactions of esters is their reaction with two equivalent of Grignard reagent to give tertiary alcohols.
When
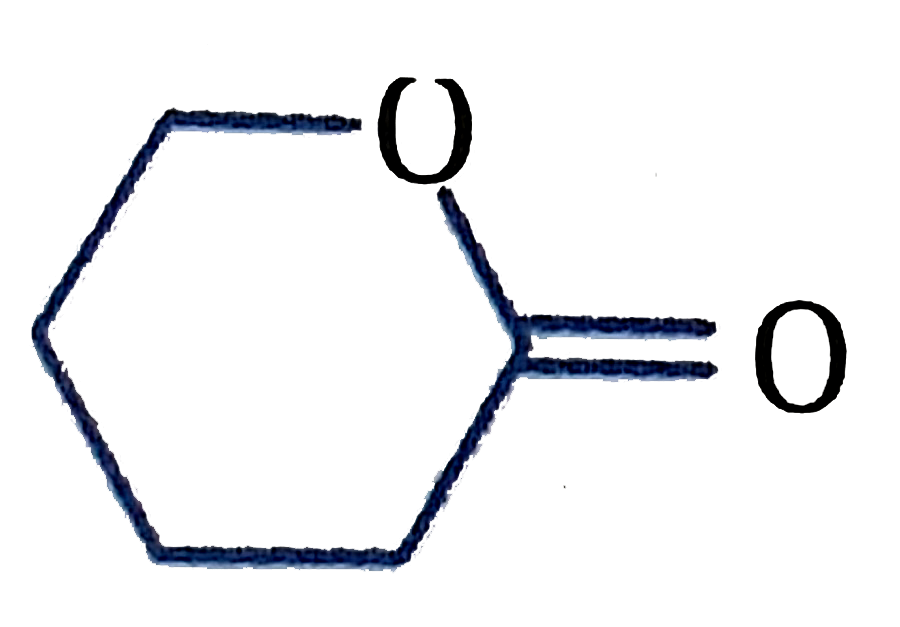
is treated with two equivalent of methyl magnesium iodide and the product acidified the final product will be
View SolutionTwo solutions X and Y have pH=4 and pH=8, respectively. Which solution will give alkaline reaction and which one acidic?
View Solution
