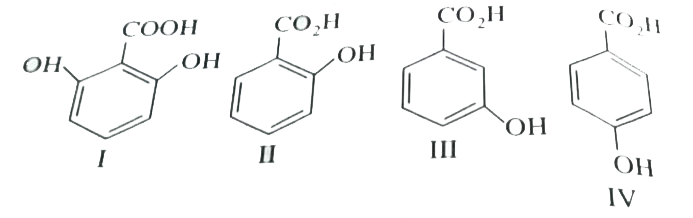
Which of the following is the correct order of stability of the following compounds ? 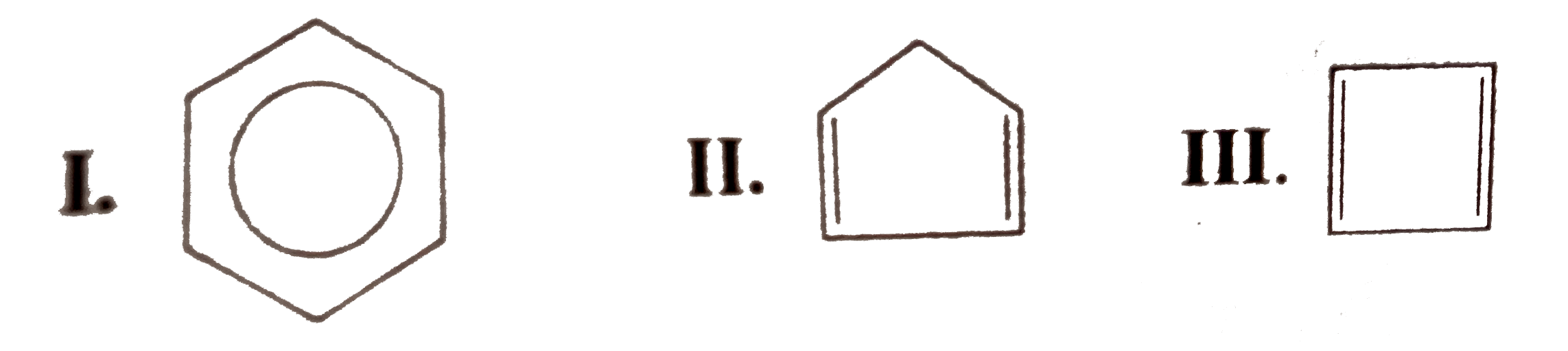
A
(I)>(II)>(III)
B
(III)>(II)>(I)
C
(II)>(I)>(III)
D
(I)>(III)>(II)
Video Solution
Text Solution
The correct Answer is:a
(I)⇒ aromatic , (II)= non− aromatic (4π¯es but not in delocalisation).
(III)⇒ anti−aromatic (4π¯es but in delocalisation). So the order of stability is :(I)>(II)>(III).
|
Similar Questions
Knowledge Check
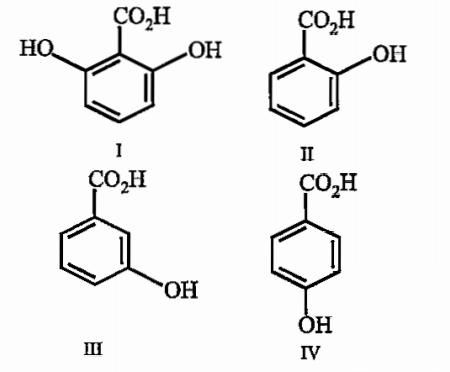
the correct order of acidity of the following compounds is
 A1>2>3B1>3>2C3>1>2D3>2>1
A1>2>3B1>3>2C3>1>2D3>2>1Which of the following is correct order of stability?
A[NiCl4]−2<[PdCl4]−2<[PtCl4]−2B[Co(H2O)6]+3<[Co(NH3)6]+3<[C(CN)6]−3C[Ni(NH3)4]+2<[Ni(CN)4]−2DAllWhich of the following is correct order of stability:
ATI3+>BI3+BPbO2>PbOCBiI5<BiF5DSn2+=Ge2+
Which of the following is the correct order of stability of the following four distinct conformation of n-butane?
View SolutionThe correct order of basicity of the following compounds is

View SolutionCorrect order of stability amont the following

View SolutionThe correct order of acidity for the following compounds is
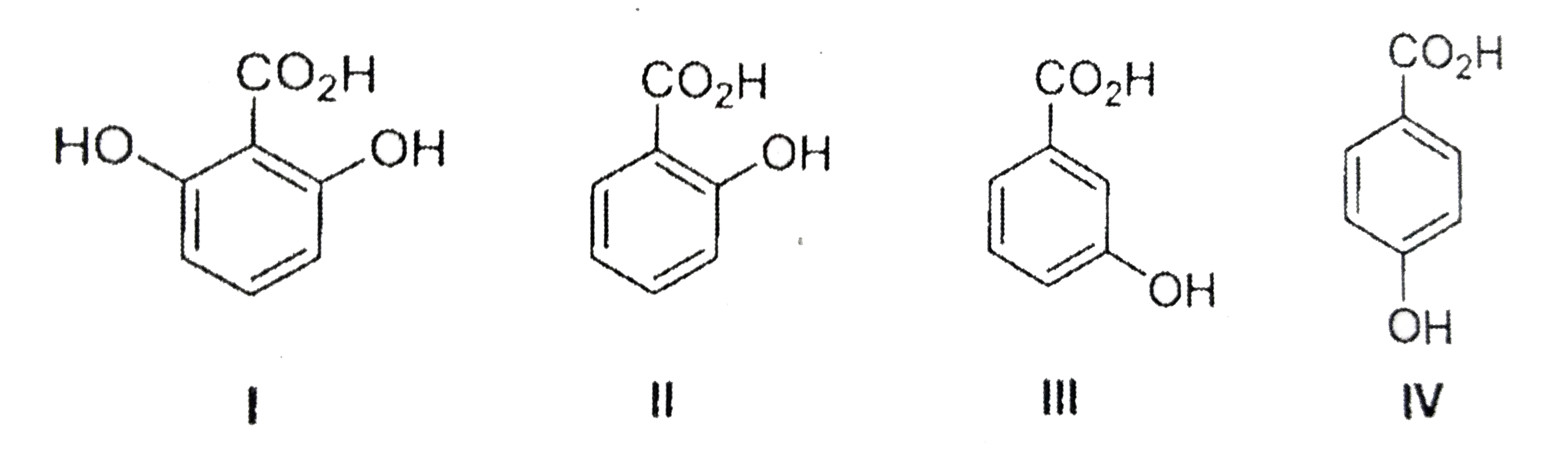
View SolutionThe correct order of acidity for the following compounds is
View Solution