In which of the following compounds does intramolecular H-bonding occure?
a. o-Nitro phenol
b, o-Cresol
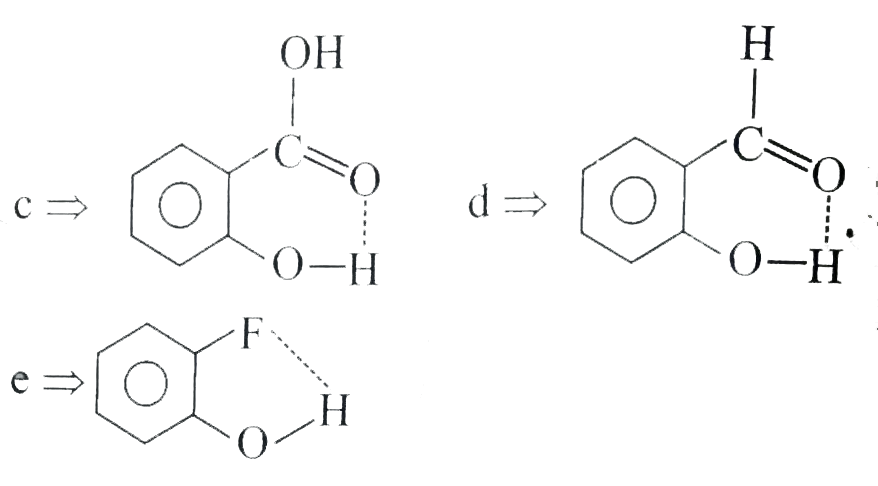
c. o-Hydrox benzoic acid
d. Salicylaldehyde
e. o-Fluoro phenol
f. o-Hydrox-benzonitrile
g. Methyl salicylate ( oil of wintergreen)
Video Solution
Text Solution
Instramolecular H-bonding occurs in (a), (c ), (d), (e), and (g).
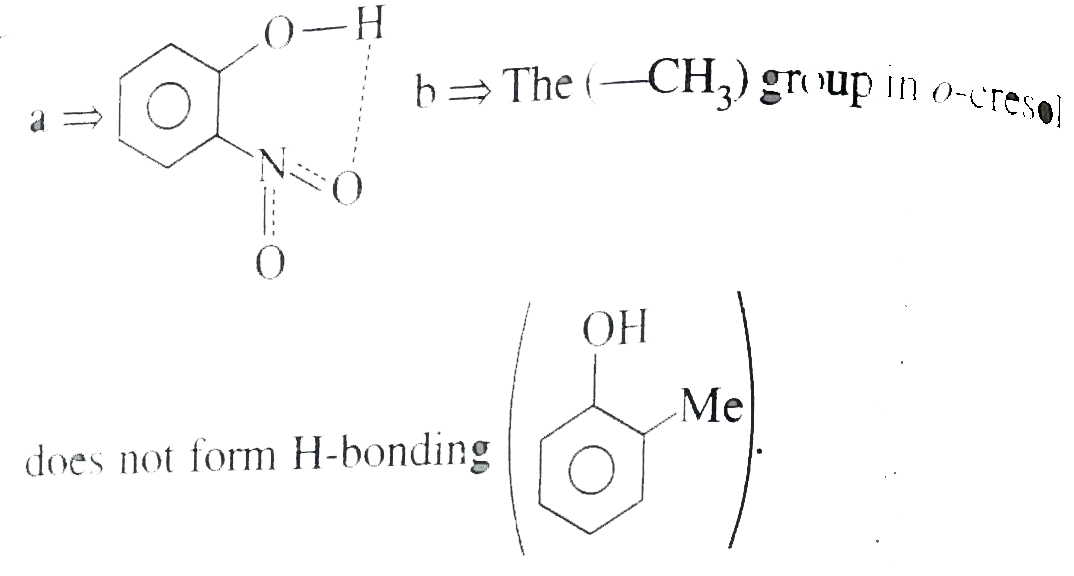
does not form H-bonding
f⇒ The (−C≡N) (sp-hydridised) is linear and hence the N-atom is too far away from the (−OH) group
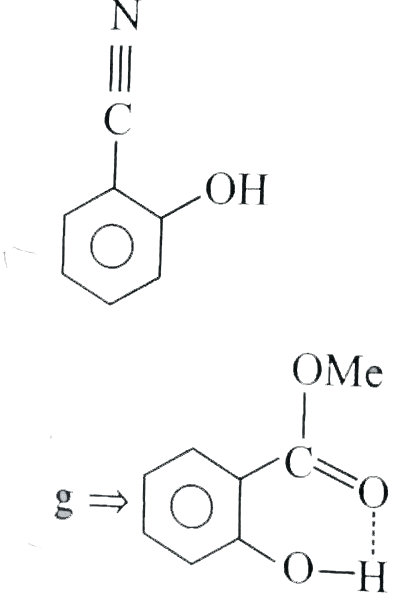
|
Similar Questions
Knowledge Check
Which of the following class of compounds does NOT contain a C=O bond ?
AKetonesBAcid anhydridesCEstersDEthersIn which of the following compounds resonance does not occurs
(a) H2O (b) SiO2 (c ) SO2 (d) CO2Aa and dBa and bCc and dDb, c and dAt which of the following reaction condition phenol gives O-phenol sulphonic acid ?
AConc. H2SO4 at high pressureBDil. H2SO4 at low temperatureCConc. H2SO4 at low temperatureDDil. H2SO4 at high temperature
Arrange the following acids in order of the increasing acidity: A= o-nitro benzoic acid, B = p-nitro benzoic acid, C= p-hydroxy benzoic acid, D= benzoic acid
View SolutionWhich of the following explanations accounts for o-nitro-phenol to be more volatile than p-nitrophenol?
View SolutionArrange the following compounds in the order of increasing acidity.
I. Benzyl alcohol II. Benzoic acid III. o-Cresol IV. Formic acid
View SolutionWhich of the following compounds exhibit chelation ?
(i) o-cresol
(ii) methyl salicylate(oil of wintergreen)
(iii) o-hydroxybenzonitrile
(iv) o-flurophenol
(v) o-iodophenol
View SolutionWhich of the following compound have larger C−O bond length than C−O bond length of H3−O∣∣C−CH3?
View Solution

