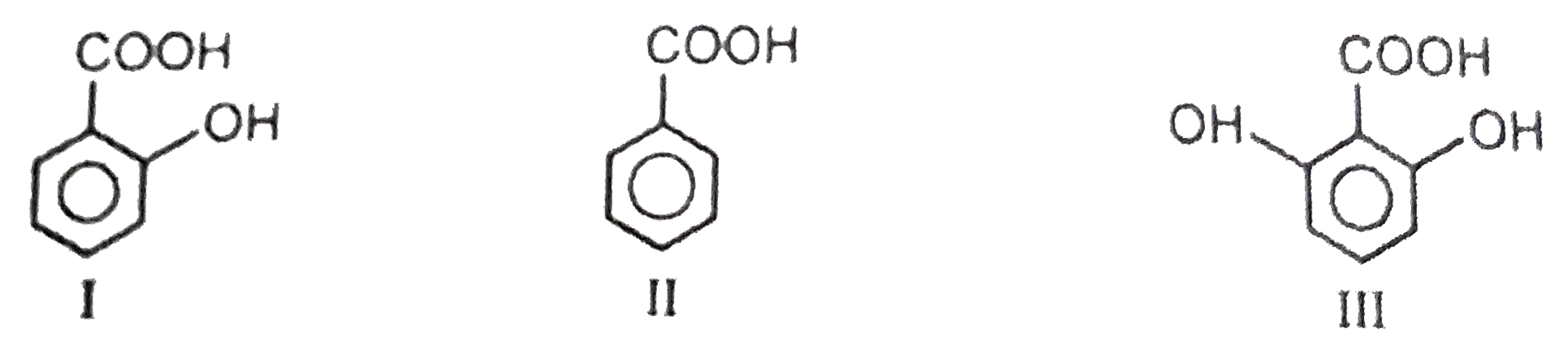
Arrange the given compouns in decreasing order of acidity and give a suitable explanation, Phenol, o-nitrophenol, o-cresol
Video Solution
Text Solution
Due to -I and -R-effect of the NO2 group, o-nitrophenol is a stronger acid than phenol but do to +I-effect of the CH3 group, o-cresol is a weaker acid than phenol. Thus, acid strength of these three phenols increases in the order: o-cresolltphenollto-nitrophenol.
|
Similar Questions
Knowledge Check
Arrange the following in decreasing order of acidity ?
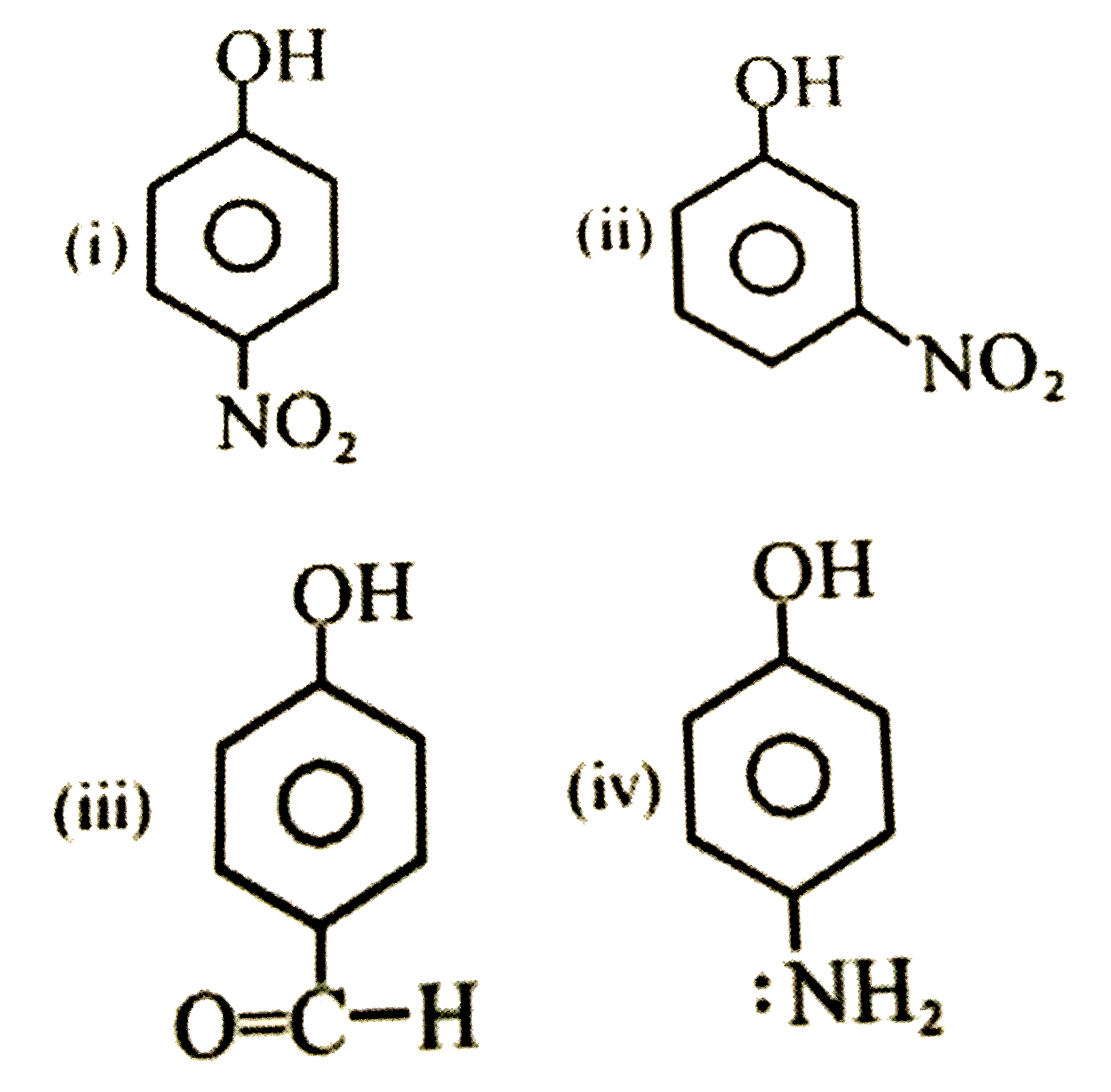 AIV>II>I>IIIBIV>II>III>ICIII>I>II>IVDI>III>II>IV
AIV>II>I>IIIBIV>II>III>ICIII>I>II>IVDI>III>II>IVArrange the followig labelled hydrogens in decreasing order of acidity :
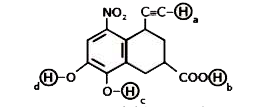 Ab>a>c>dBb>c>d>aCc>b>d>aDc>b>a>d
Ab>a>c>dBb>c>d>aCc>b>d>aDc>b>a>d
Arrange the following in decreasing order of acidic strength
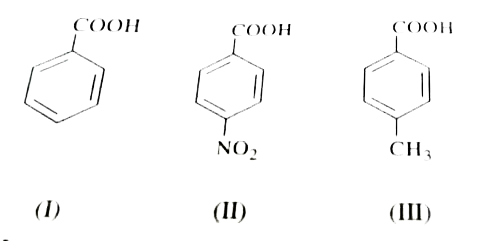
View SolutionArrange hypohalous acids in decreasing order of acidic nature.
View SolutionArrange the following compounds in decreasing order of acidity.
H2O,ROH,HC≡CH
View SolutionArrange the following compounds in decreasing order of acidity :
H2O,ROH,HC≡CH
View SolutionArrange the following compounds in increasing order of acidic strength :
p-nitrophenol, ethanol, phenol
View SolutionArrange the following compounds in decreasing order of acidity.
H2O,ROH,HC≡CH
View Solution