Statement-1 The ratio of σ-bonds and π-bonds in tetra-cynomethane is 1.
Statement-2 Tetra-cyanomethane has 8π and 8σbonds.
A
If both the statement are TRUE and Statement -2 is the correct explanation of Statement-1:
B
If both the statement are TRUE but Statement-2 is not the correct explanation of Statement-1
C
If statement-1 is TRUE and Statement-2 is FALSE
D
If statement -1 is FALSE and Statement-2 is TRUE
Video Solution
Text Solution
The correct Answer is:A
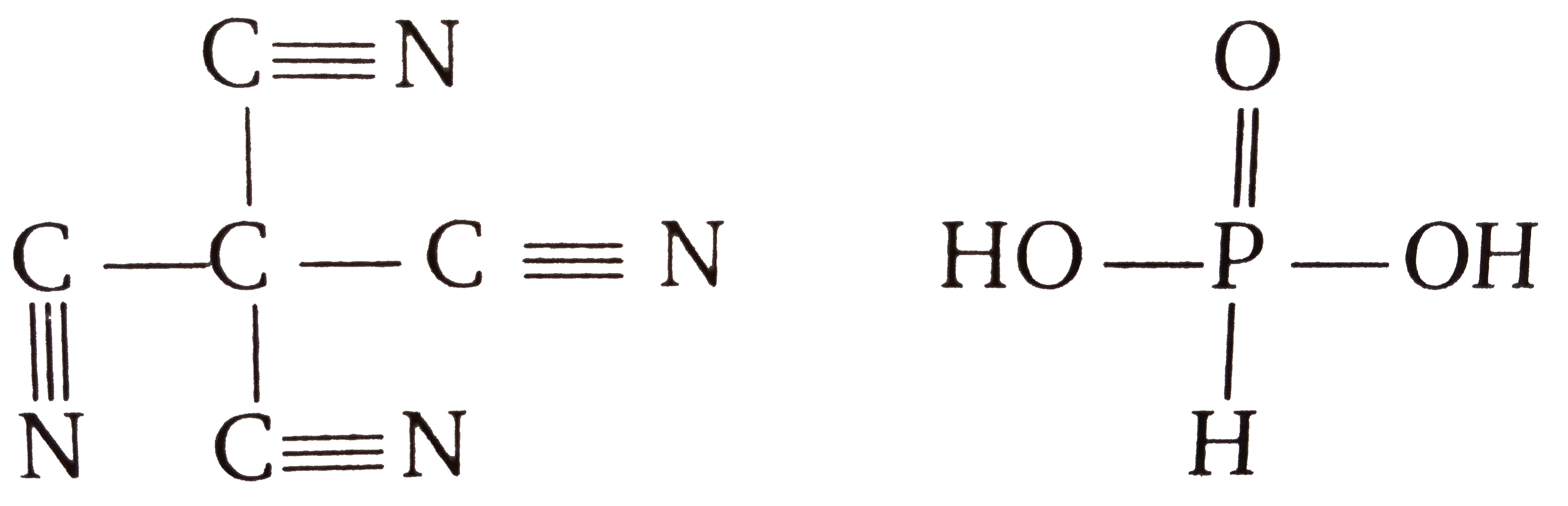
(S&E are correct and E is correct explanation of S)
|
Similar Questions
Knowledge Check
The ratio of σ− bond and π− bond in tetracryano ethylene is :
A2:1B1:1C1:2DNone of theseThe ratio of sigma and pi bonds in benzene is
A4:1B2:3C6:1D1:1The ratio of σandπ bond in benzene is
A2B4C6D8
