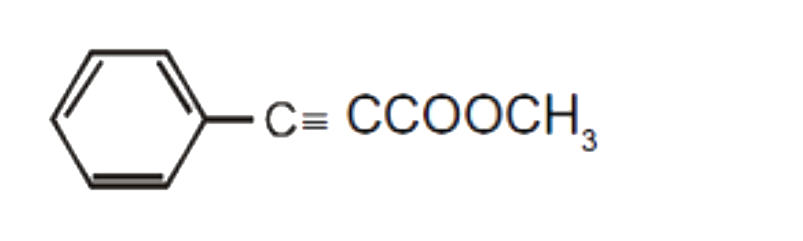Sum of σ and π bonds present in tetra cyano ethylene is
Video Solution
|
Answer
Step by step video & image solution for Sum of sigma and pi bonds present in tetra cyano ethylene is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Number of σ and π bonds present in :
A10σ,3πB10σ,2πC9σ,2πD8σ,3πHow many σandπ bonds are present in tetra cyanoethylene ?
ANine σ and nine πBFive π and nine σCNine σ and seven πDEight σ and eight πThe ratio of σ− bond and π− bond in tetracryano ethylene is :
A2:1B1:1C1:2DNone of these
Total number of sigma and π bonds present in ethyne are
View SolutionHow many sigma and pi bonds are there in tetracyano ethylene molecule?
View SolutionThe number of sigma (σ) and pi (π) bonds present in acetylene are _____ respectively.
View SolutionIn the following questions, a statement of assertion (A) is followed by a statement of reason (R )
A : Equal number of sigma and π bonds are present in ethyne.
π Bond is stronger than σ bond.
View SolutionThe number of σ−andπ bonds present in pent-4en-1-yne is :
View Solution