order of reaction is
never zero
never fractional
always equal to number of molecules taking part in reaction
an experimentally determined quantity
The correct Answer is:D
Step by step video, text & image solution for order of reaction is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL KINETICSBOOK - MARVEL PUBLICATIONCHAPTER - CHEMICAL KINETICSEXERCISE - TEST YOUR GRASP
50 Videos
- BIOMOLECULESBOOK - MARVEL PUBLICATIONCHAPTER - BIOMOLECULESEXERCISE - TEST YOUR GRASP
40 Videos
- CHEMICAL THERMODYNAMICS AND ENERGETICSBOOK - MARVEL PUBLICATIONCHAPTER - CHEMICAL THERMODYNAMICS AND ENERGETICSEXERCISE - MULTIPLE CHOICE QUESTIONS (TEST YOUR GRASP)
50 Videos
Similar Questions
Knowledge Check
Which one the following statement for order of reactions is not correct ?
AOrder can be detemined experimentallyBOrder of reaction is equal to sum of powrs of concentration temrs in differential rate law.CIt is not affected by the by the stoichiometric coefficient of the reactants.DOrder cannot be fractional.Which of the following is incorrect about the order of reaction ?
AIt is calculated experimentallyBIt is sum of powers of concentrations in rate law expressionCThe order of reaction cannot be fractionalDThere is not necessarily a connection between order and stoichiometry of a reactionWhich of the following is not used to find the order of reaction ?
AGraphical methodBOstwald dilution law methodCIsolation methodDIntegrated method
Arrange the following in increasing order of reaction with HCN
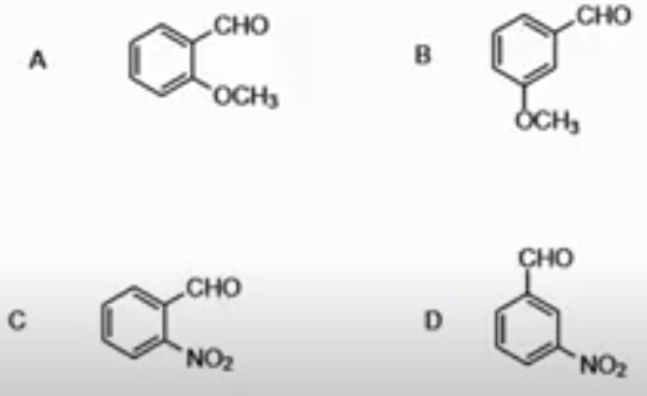
View SolutionDefine 'order of a reaction'.
View SolutionThe order of reaction may be a fractional number.
View SolutionWhich of the following statements is correct regarding order of reaction
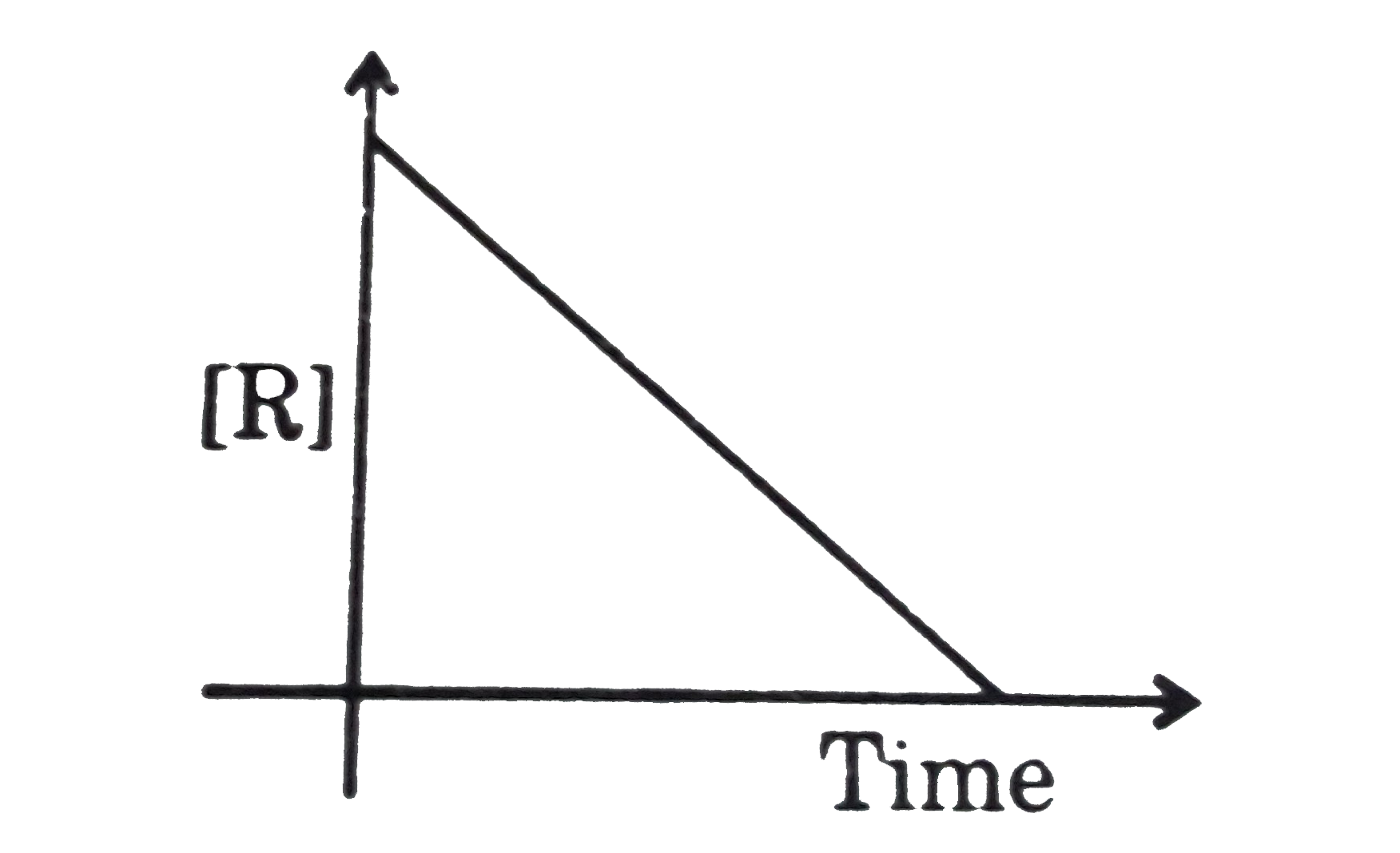
View SolutionAdjoining graph is for a reaction which have only single reactant (R) and order of reactions depends only on that single reactant then possible order of reactions depends only on that single reactant then possible order of reaction may be:
View Solution

