Ketone never show
A
chain isomerism
B
position isomerism
C
geometrical isomerism
D
optical isomerism
Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NIKITA PUBLICATIONChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:SECTION - II : CARBOXYLIC ACIDS
Explore 198 Videos
- ALCOHOLS , PHENOLS AND ETHERS Book:NIKITA PUBLICATIONChapter:ALCOHOLS , PHENOLS AND ETHERS Exercise:MCQs
Explore 589 Videos
- BIOMOLECULES Book:NIKITA PUBLICATIONChapter:BIOMOLECULES Exercise:MCQs
Explore 428 Videos
Similar Questions
Knowledge Check
Which of the following elements never show positive oxidation number?
AOBFeCGaDF[A]: The vascular cambium is absent in monocots.
[R]: The monocots never show secondary growthAIf both A and R are true and R is the correct explanation of ABIf both A and R are true but R is not the correct explanation of ACIf A is true and R is falseDIf both A and R are falseKetones do not show reducing property because
Athey do not behave as reducing agentsBthey are not oxidizedCof absence of oxidisable H-atom on carbonyl groupDthey are very stable
The element of Group 16, which never shows negative oxidation state is
View SolutionKetones are isomeric with
View SolutionKetone (A) NH2OH.HCl−−−−−−−−→ (B) H⊕−−→
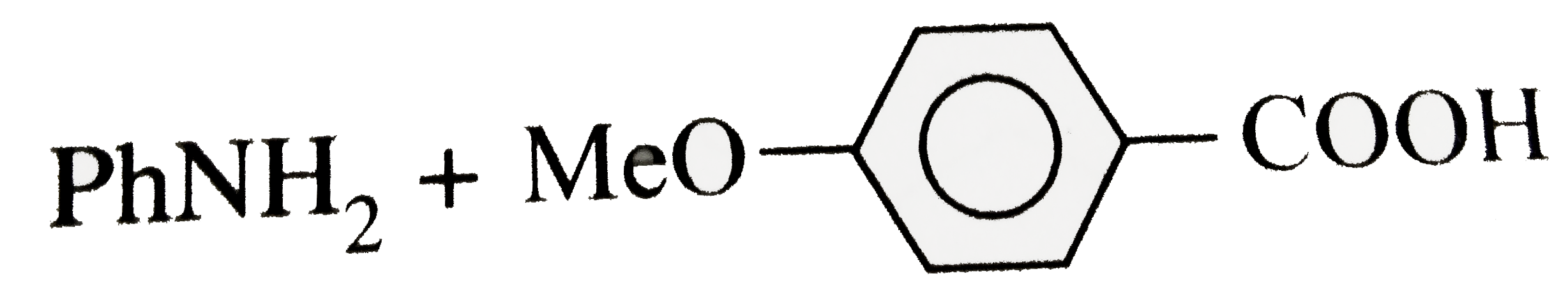
The ketone (A) and compound (B), respectively, are:
Ketone (A)
I.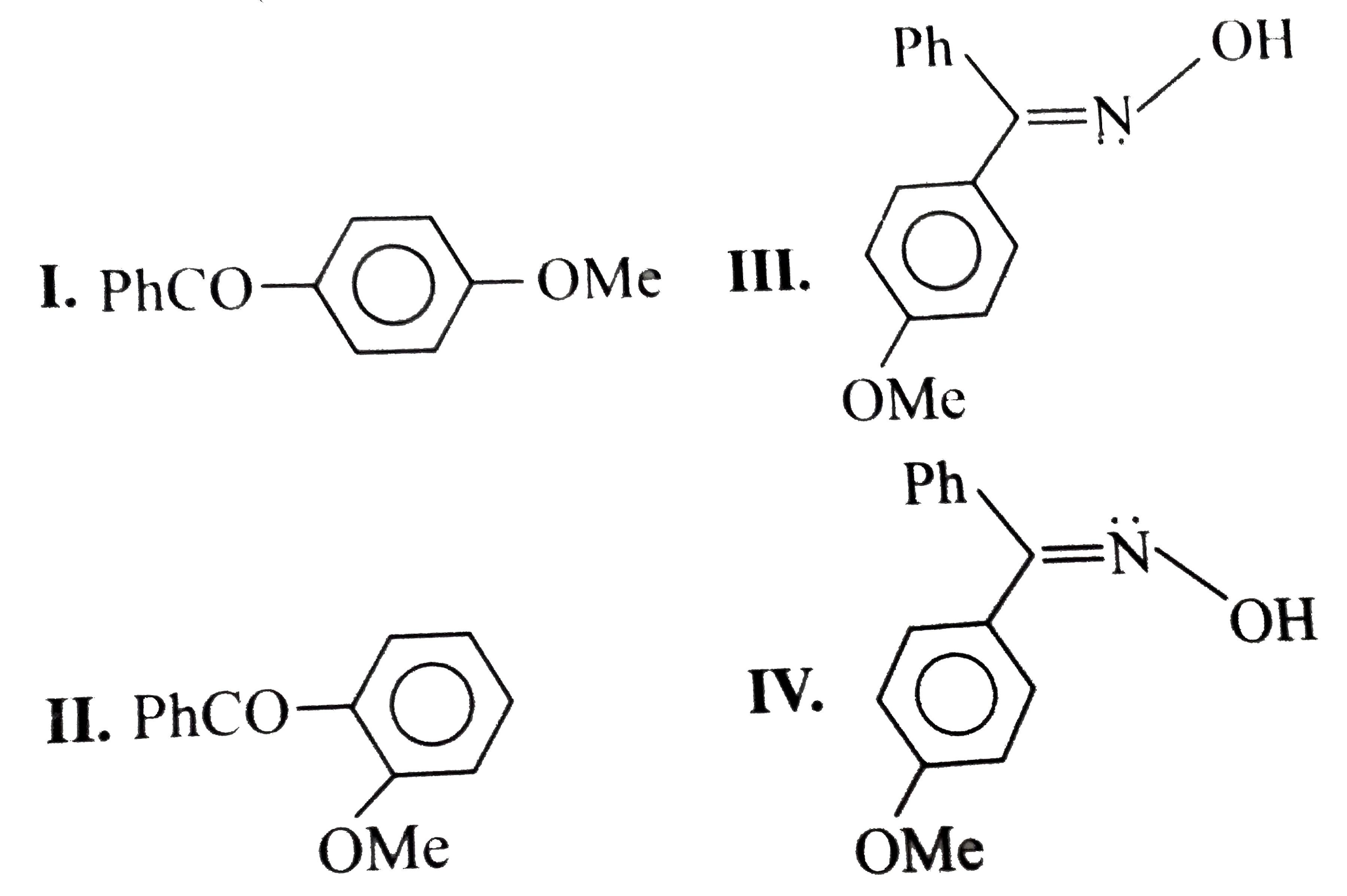
Compound (B)
View SolutionMonocarboxlic acid shows functional isomerism with
(1) Ester (2) aldehydes
(3) ketones (4) ethers
View SolutionAssertion: Oximes are less acidic than hydroxyl amine.
Reason: Oximes of aldehydes and ketones show geometrical isomerism.
View Solution

