The π - bond in carbonyl group is formed by
A
s-s-overlapping
B
p-p- overlapping
C
s-p- overlapping
D
p-d- overlapping
Text Solution
The correct Answer is:B
|
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBook:NIKITA PUBLICATIONChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDSExercise:SECTION - II : CARBOXYLIC ACIDS
Explore 198 Videos
- ALCOHOLS , PHENOLS AND ETHERS Book:NIKITA PUBLICATIONChapter:ALCOHOLS , PHENOLS AND ETHERS Exercise:MCQs
Explore 589 Videos
- BIOMOLECULES Book:NIKITA PUBLICATIONChapter:BIOMOLECULES Exercise:MCQs
Explore 428 Videos
Similar Questions
Knowledge Check
π bond is formed by
Aoverlapping of atomic orbitals on the axis of nucleiBmutual sharing of pi electronCsidewise over lapping of half filled p-orbitalsDoverlapping of s-orbitals with p-orbitlasThe carbon atom of carbonyl group is
Asp3- hybridisedBsp2 - hybridisedCsp - hybridisedDsp3d- hybridisedCarbonyl group undergoes
Aelectrophilic addition reactionsBnucleophilic addition reactionCbothDcan not predict
Assertion The dC−O in bridging carbonyl geroup is longer than that of terminal carbonyl group
Reason With increase in extent of synergic bonding the C−O bond length increases .
View SolutionThe carbon atom of the carbonyl group is :
View SolutionThe M-C π bond is formed by the
View SolutionDuring Clemmensen reduction, a carbonyl group is converted into
View SolutionAssertion (A): Pi bond is never formed alone. It is formed along with a sigma bond
Reason (R): Pi bond is formed by sideway overlap of p- orbitals only.
View Solution


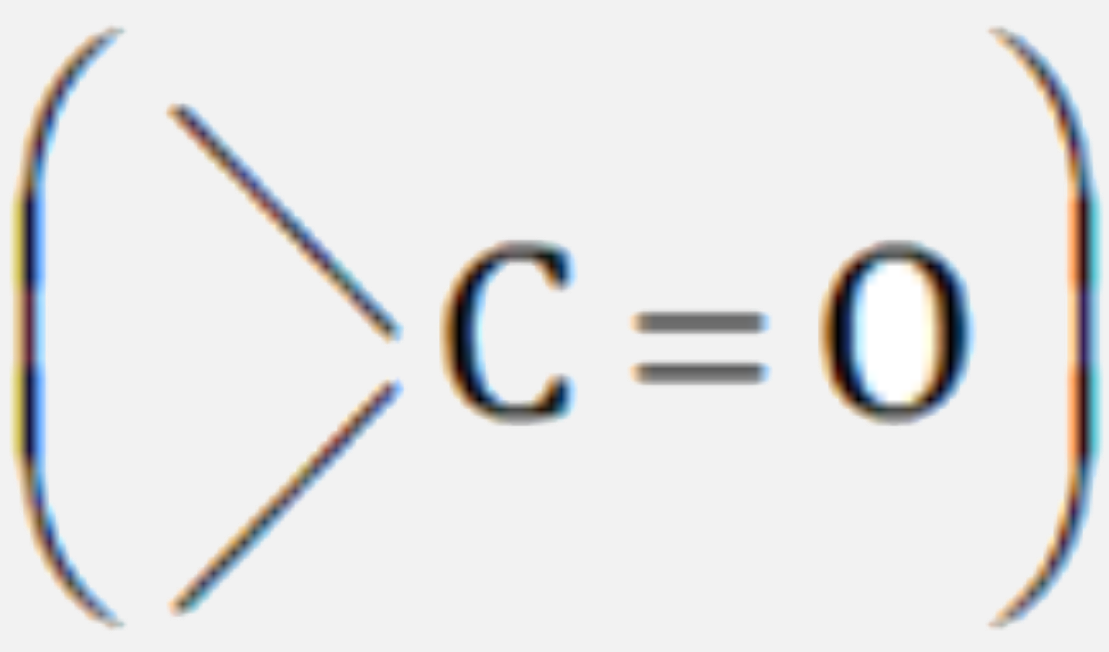 is suppressed the most in
is suppressed the most in