Carbon suboxide (C3O2)
is obtained by dehydrating malonic acid with P2O5
is a linear molecule
is a foul smelling gas
all the above are correct
The correct Answer is:D
All the above statements are correct.
Topper's Solved these Questions
- P BLOCK ELEMENTS ( Group 13 -14)Book:A2ZChapter:P BLOCK ELEMENTS ( Group 13 -14)Exercise:Section B - Assertion Reasoning
Explore 21 Videos
- P BLOCK ELEMENTS ( Group 13 -14)Book:A2ZChapter:P BLOCK ELEMENTS ( Group 13 -14)Exercise:AIPMT/ NEET Questions
Explore 15 Videos
- P BLOCK ELEMENTS ( Group 13 -14)Book:A2ZChapter:P BLOCK ELEMENTS ( Group 13 -14)Exercise:General Characterstics, Pysical And Chemical Properties (Group 14)
Explore 49 Videos
- MOCK TESTBook:A2ZChapter:MOCK TESTExercise:Mock Test 2
Explore 45 Videos
- REDOX REACTIONSBook:A2ZChapter:REDOX REACTIONSExercise:Section D - Chapter End Test
Explore 29 Videos
Similar Questions
Knowledge Check
Carbon suboxide (C2O2) has recently been shown as a component of the atmosphere of venus. Which of the following formulation represents the correct ground state Lewis structure for C3O2 ?
A:O:C:C::O:B:O:C::C:O:C:O::C::C::C::O:D:O::C:C::O:Carbon suboxide (C3O2) is a foul-smelling gas. Which of the following formulation represents the correct ground state Lewis structure for carbon suboxide?
A:O:C:C:C:O:B:O::C::C::C::O:C:O:C::C:C:OD:..O::C::C::C::..O:Carbon suboxide (C3O2) has recently been shown as a component of the atmosphere of Venus. Which of the following formulation represents the correct ground state Lewis structure of carbon suboxide?
A:O:C::C:C:O:B:..O::C::C:C::O:C:..O::C::C::C::..O:D:O:C:C:C:O:
Carbon suboxide CO3O2(O=C=C=O) is obtaind as a corlurless gas by the dehyration of malonic acid with .
View SolutionC3H6O2
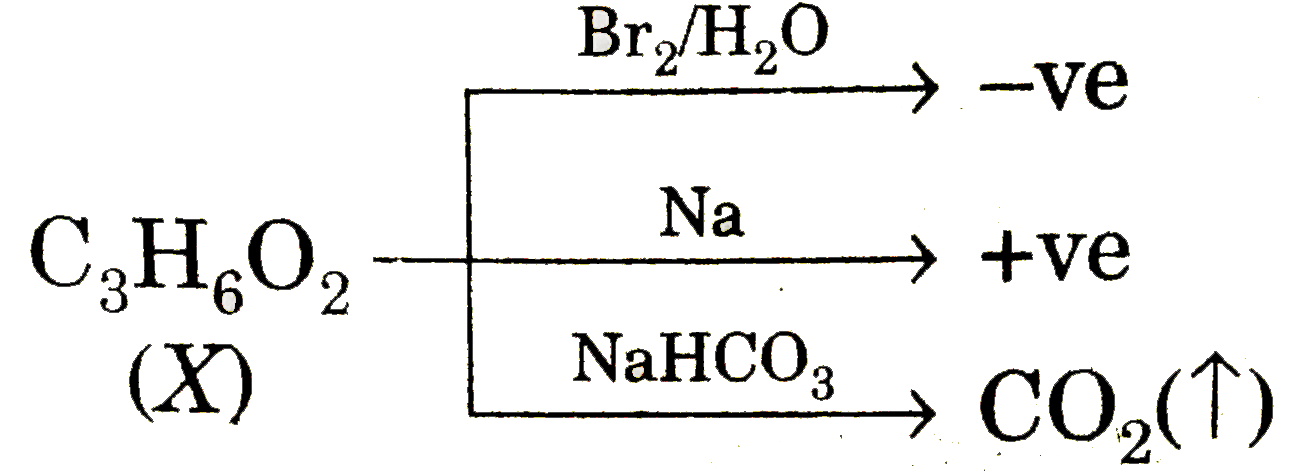
View SolutionIn pyro metallurgy the metals are extracted from ores and concentrates by heat treatment. This involves conversion to metal oxide. This is followed by the reduction of the oxide to the metal using carbon or carbon monoxide.
4Cr(s)+3O2(g)→4Cr2O3(s),
ΔG∘=1500kJ/mol at1200∘C
2Mn(s)+O2(g)→2MnO(s),
ΔG∘=−600kJ/mol at1200∘C
2C(s)+O2(g)→2CO(g),
ΔG∘=−550kJ/mol at1200∘C
View SolutionAssertion: C3O2 has linear structure.
Reason: Each carbon atom in C3O2 in sp -hydridized.
View SolutionWhat is the oxidation number of carbon in C3O2 ( carbon suboxide ) ?
View Solution
