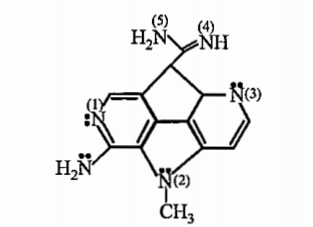
The correct increasing order of basic strength of the labeled N is:
A
I<II<III<IV
B
I<II<IV<III
C
I<III<IV<II
D
IV<III<II<I
Video Solution
Text Solution
The correct Answer is:B
III is most basic nitrogen because protonation here produces resonance stabilised conjugate acid.

I is least basic as lone pair of nitrogen is delocalized over two carbonyl groups. II is less basic than IV because lone pair of nitrogen II is more delocalized, less available for donation.
|
Similar Questions
Knowledge Check
The correct increasing order of acidic strength is
ACH3OH>H2O>(CH3)2CHOHBH2O>CH3OH>(CH3)2CHOHC(CH3)2CHOH>CH3OH>H2ODH2O>(CH3)2CHOH>CH3OH
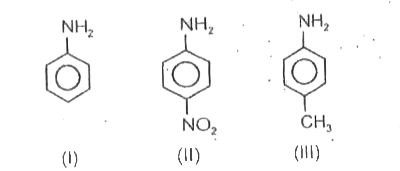
Correct order of basic strengthAI>II>III>IVBII>III>I>IVCIII>II>I>IVDIV>I>II>IIIThe correct increasing order of basic strength of the following compounds is……
 AII<III<IBIII<I<IIICIII<II<IDII<I<III
AII<III<IBIII<I<IIICIII<II<IDII<I<III
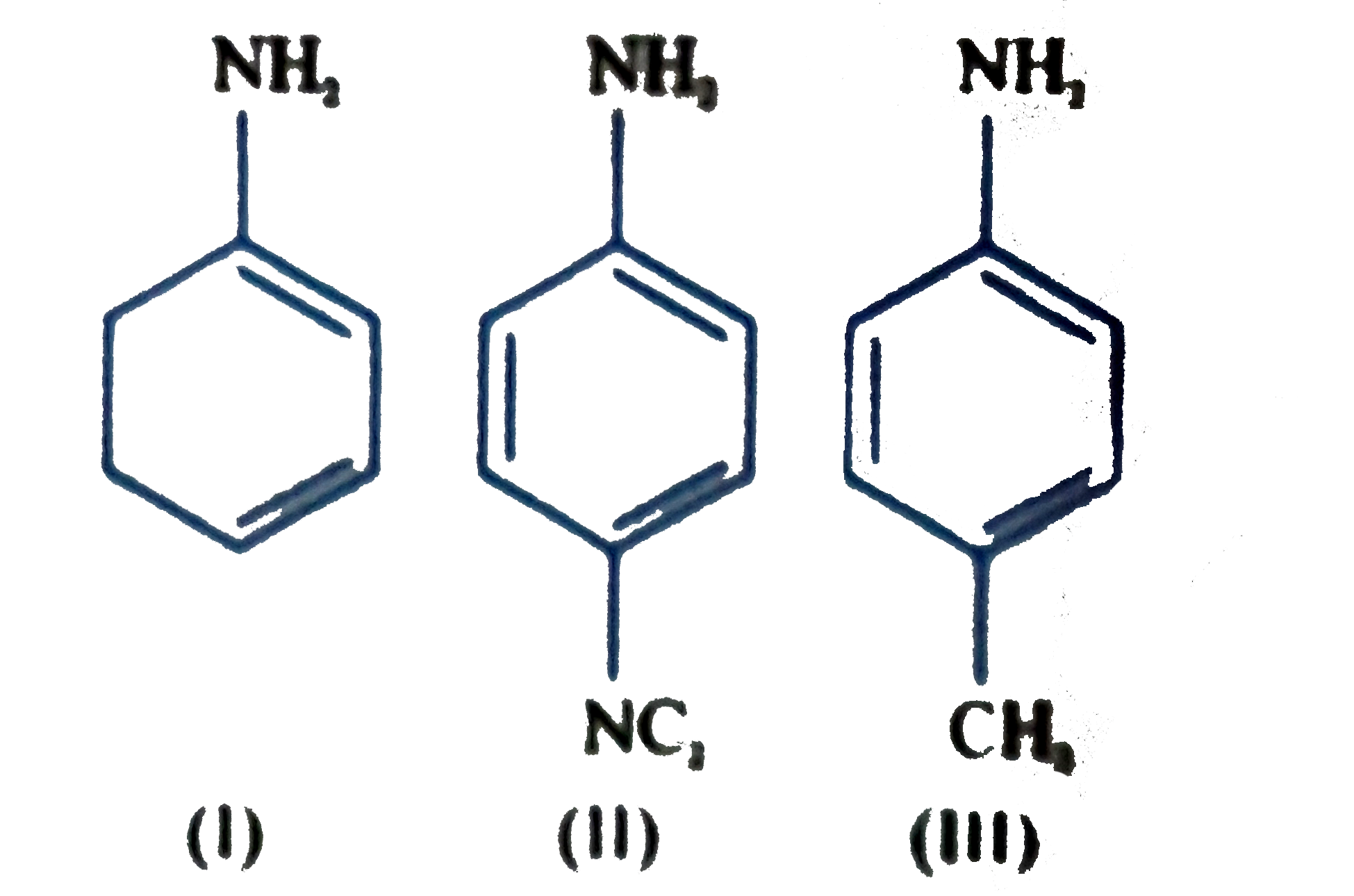
The correct increasing order of basic strength for the following compounds is
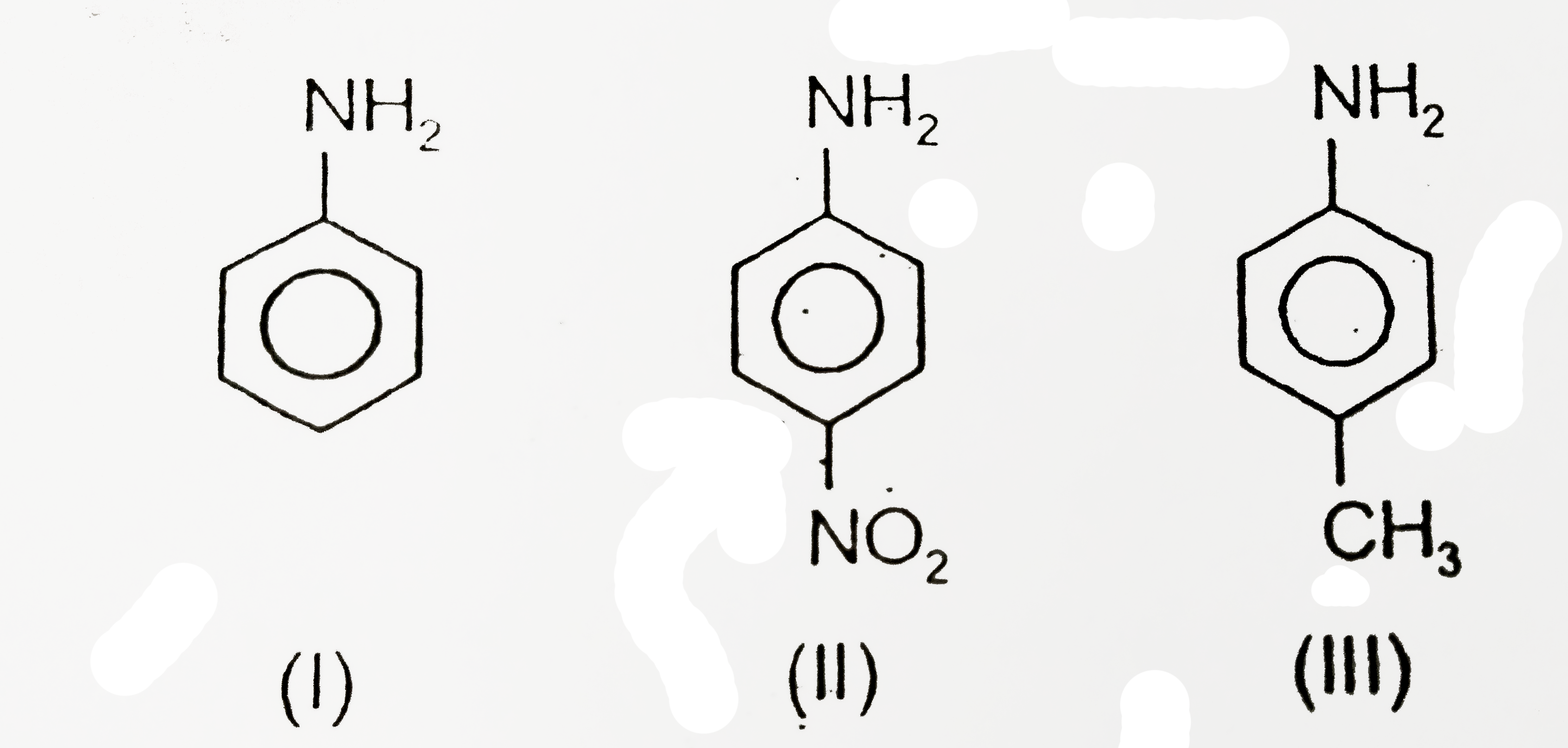
View SolutionThe correct increasing order of basic strength for the following compounds is
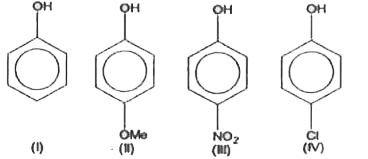
View SolutionGive the correct increasing order of acid strength.
View SolutionThe correct increasing order of basic strength for the following compounds is ________.
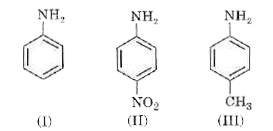
View SolutionThe correct increasing order of basic strength for the following compounds is
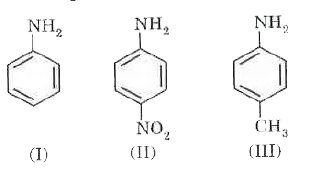
View Solution