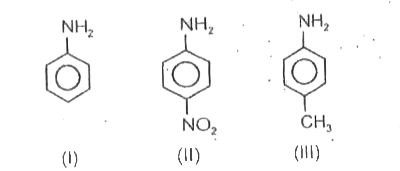
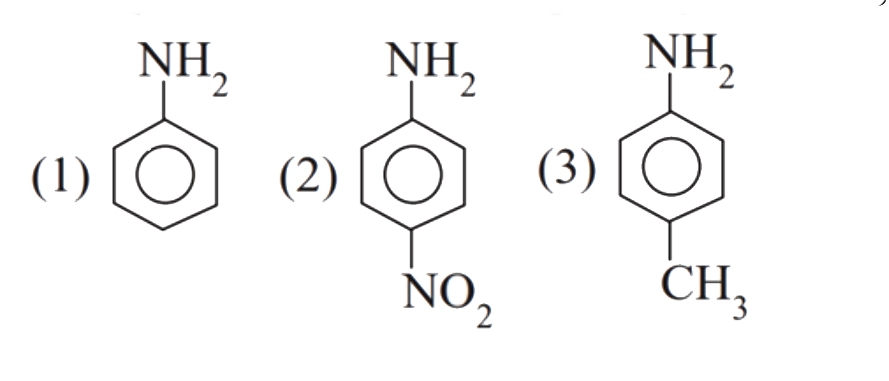
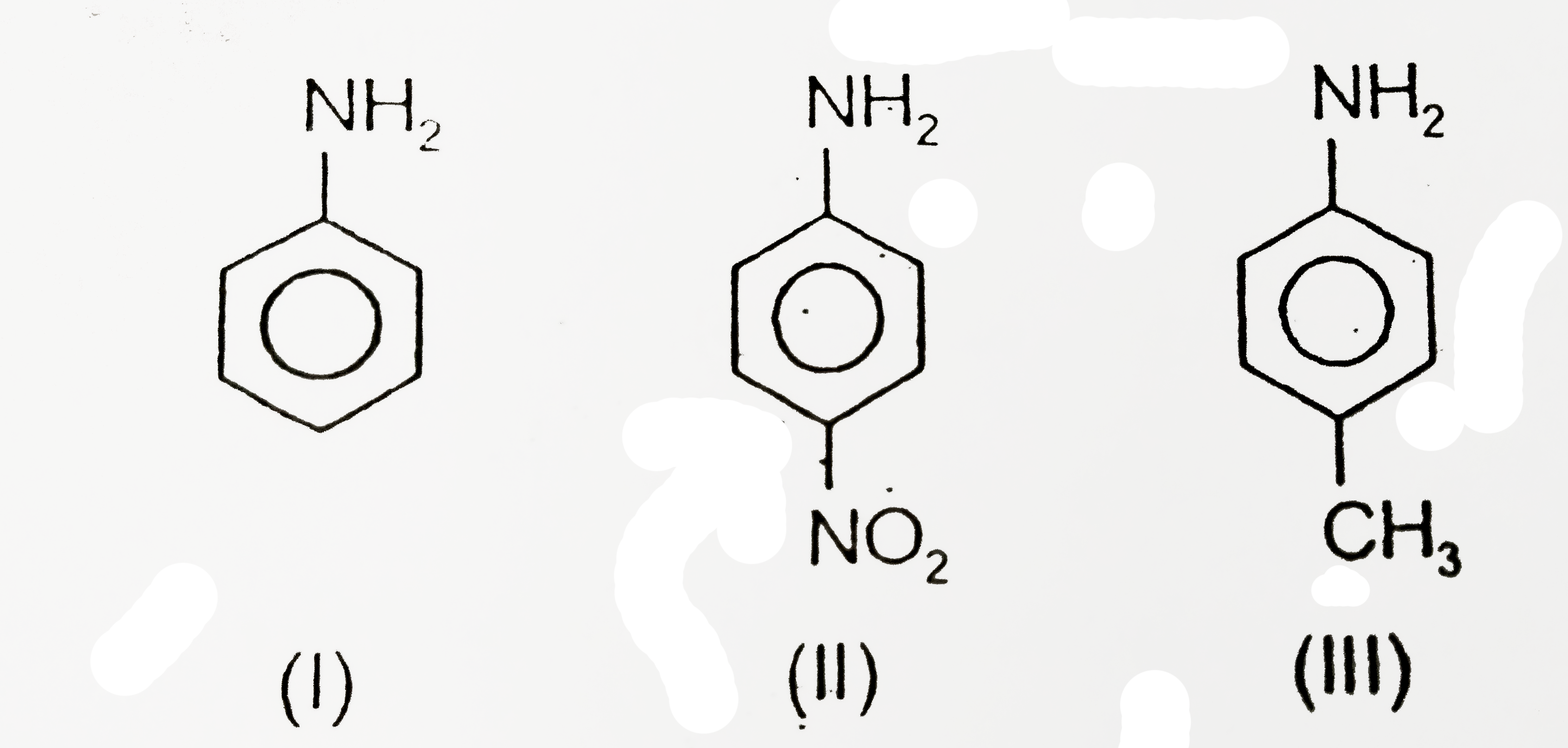The correct increasing order of basic strength of the following compounds is……
A
II<III<I
B
III<I<III
C
III<II<I
D
II<I<III
Video Solution
Text Solution
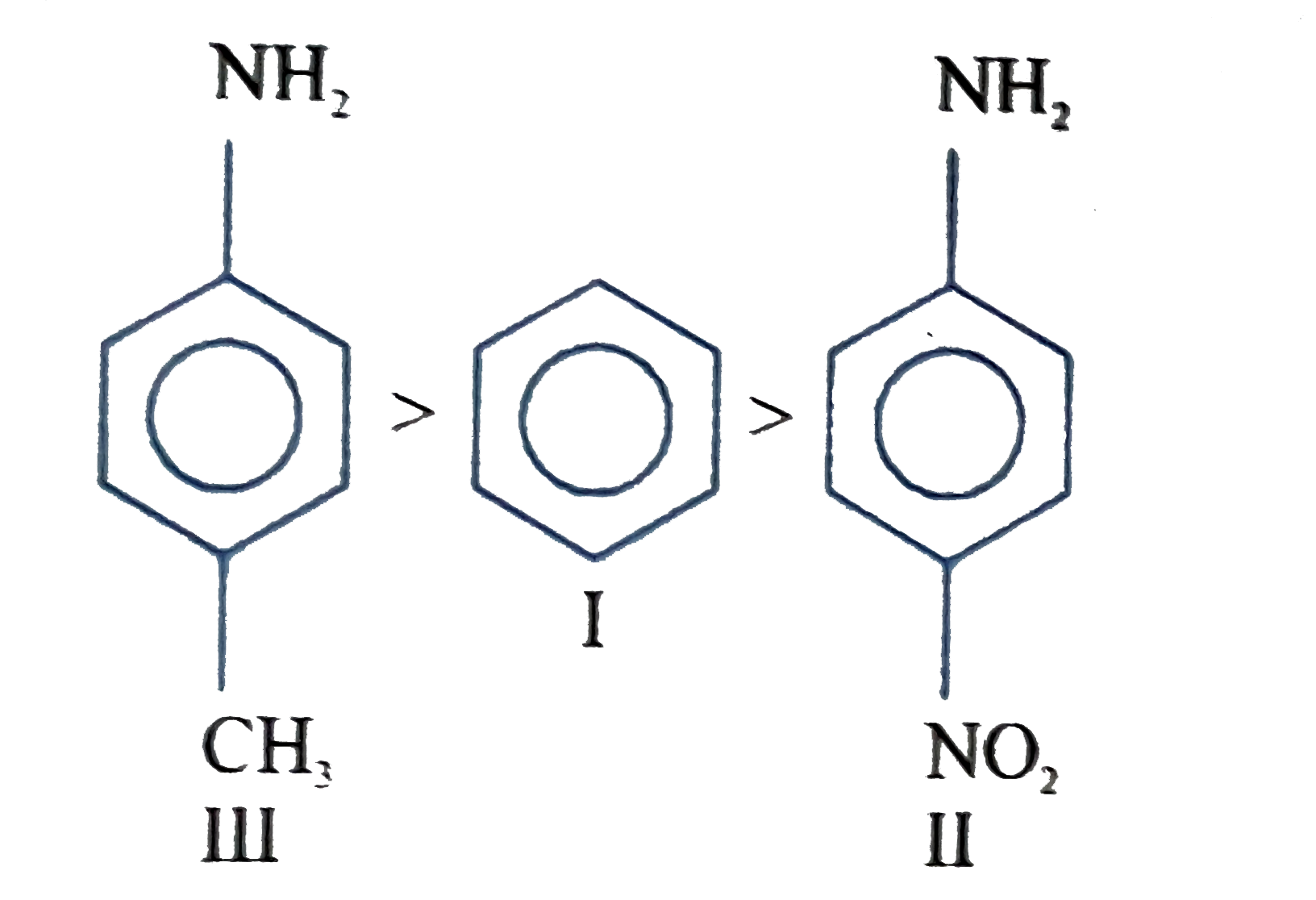
The correct Answer is:D
The correct increasing order of basic strength is as follow
Greater the electron density towards ring after will be its basic strength. Electron withdrawing group decreases basic strength while electron donating group increases basic strength.
|
Similar Questions
Knowledge Check
The correct increasing order of basic strength for the following compounds is
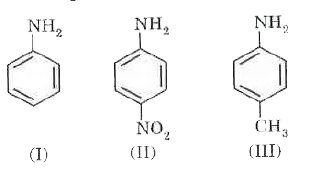 AIII < I < IIBIII < II < ICII < I < IIIDII < III < I
AIII < I < IIBIII < II < ICII < I < IIIDII < III < IThe correct increasing order of basic strength for the following compounds is
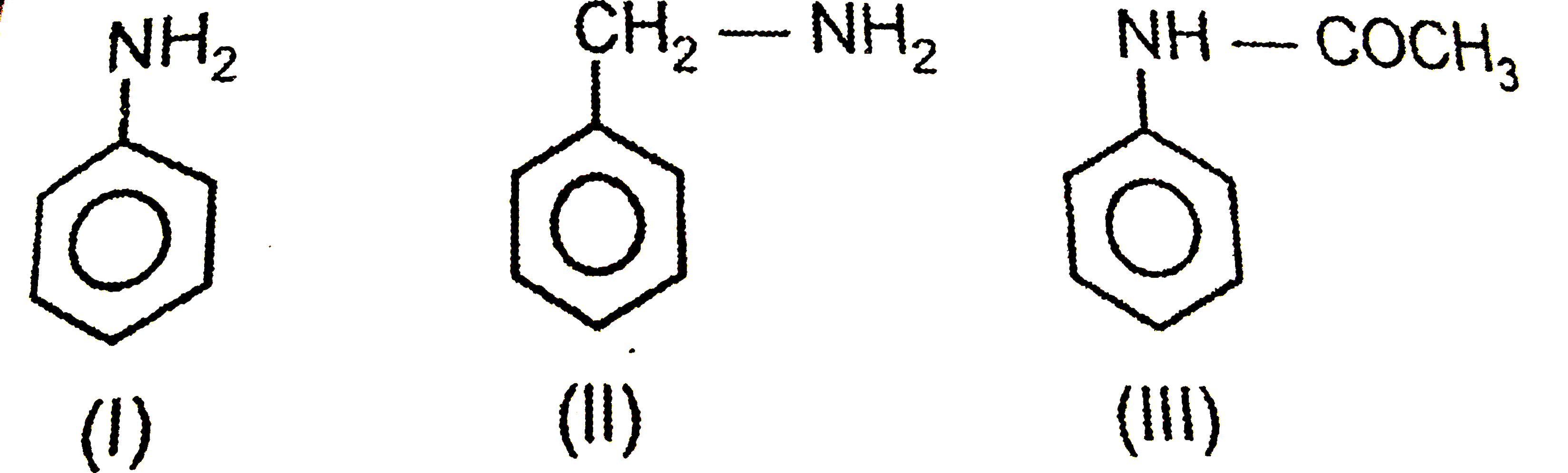 AIgtIIgtIIIBIIIgtIgtIICIIgtIgtIIIDIIgtIIIgtI
AIgtIIgtIIIBIIIgtIgtIICIIgtIgtIIIDIIgtIIIgtIThe correct increasing order of basic strength for the following compounds is
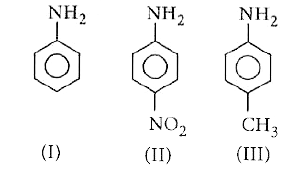 AIII<I<IIBIII<II<ICII<I<IIIDII<III<I
AIII<I<IIBIII<II<ICII<I<IIIDII<III<I
The correct increasing order of basic strength for the following compounds is
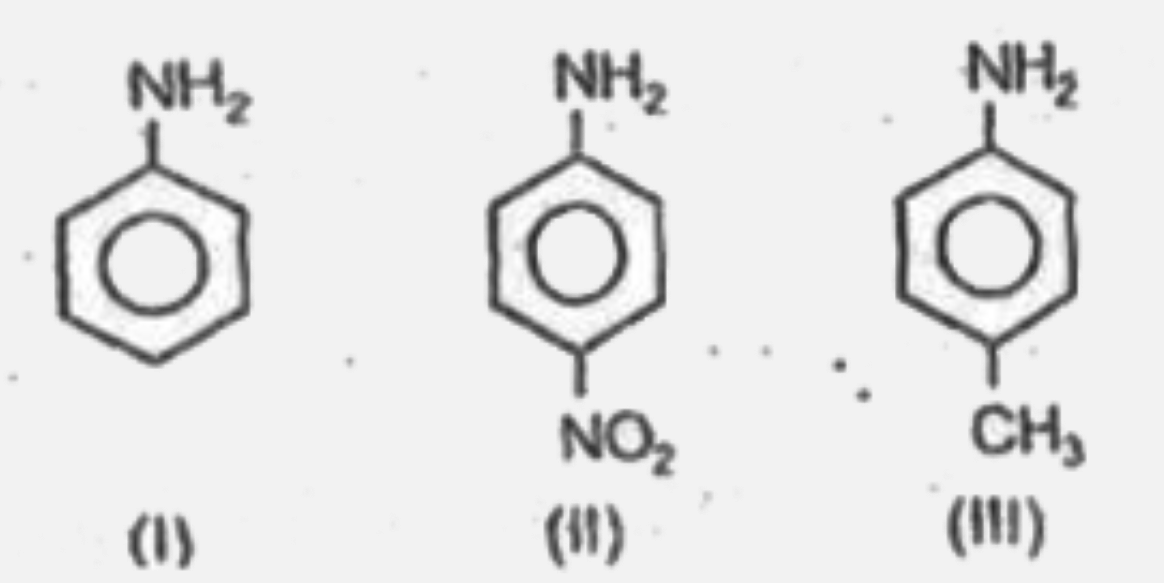
View SolutionThe correct increasing order of basic strength for the following compounds is
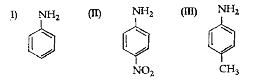
View SolutionThe correct increasing order of basic strength for the following compounds is ____
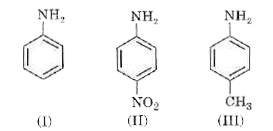
View SolutionThe correct increasing order of basic strength for the following compounds is ________.
View SolutionThe correct increasing order of basic strength for the following compounds is:
View Solution