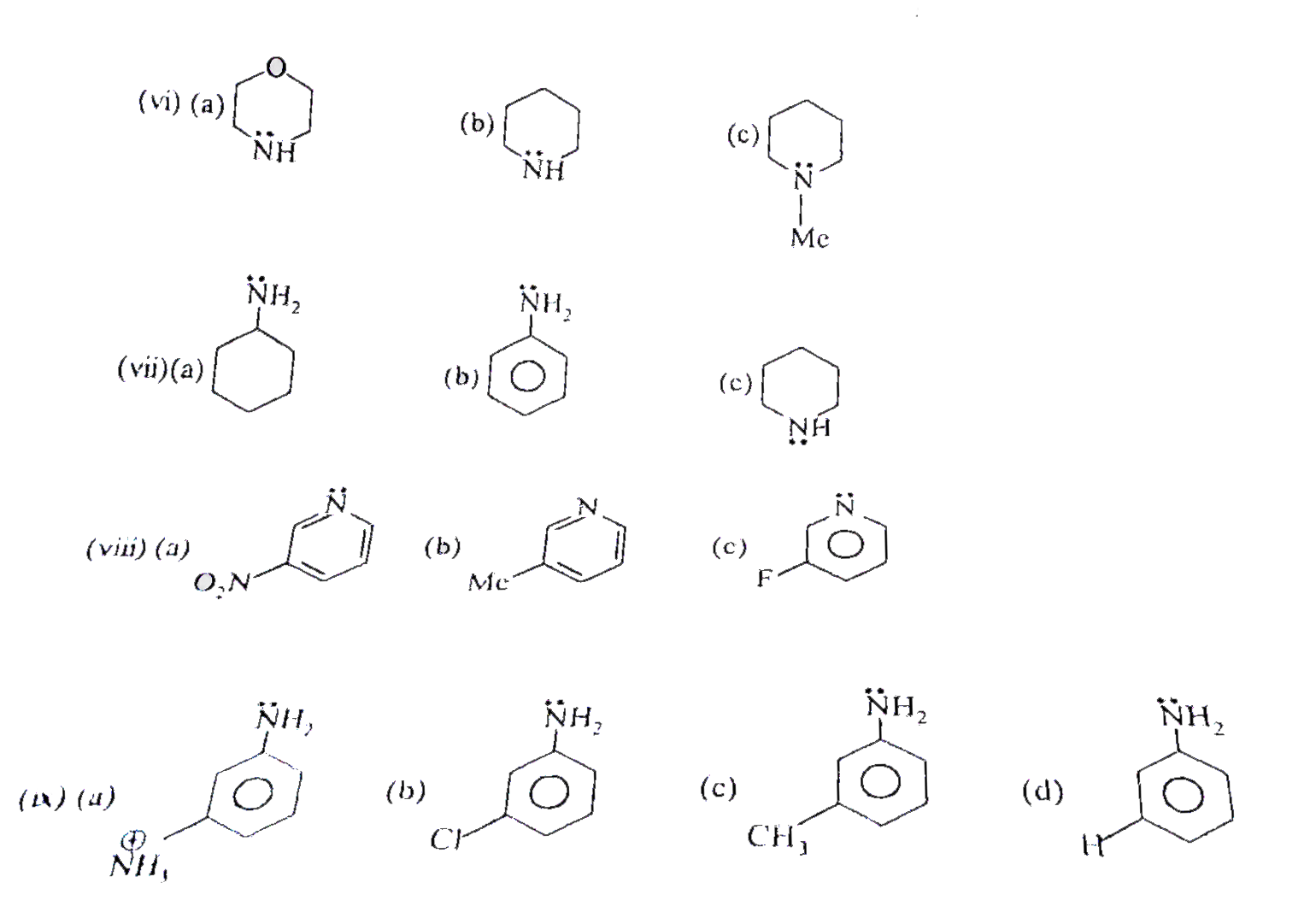
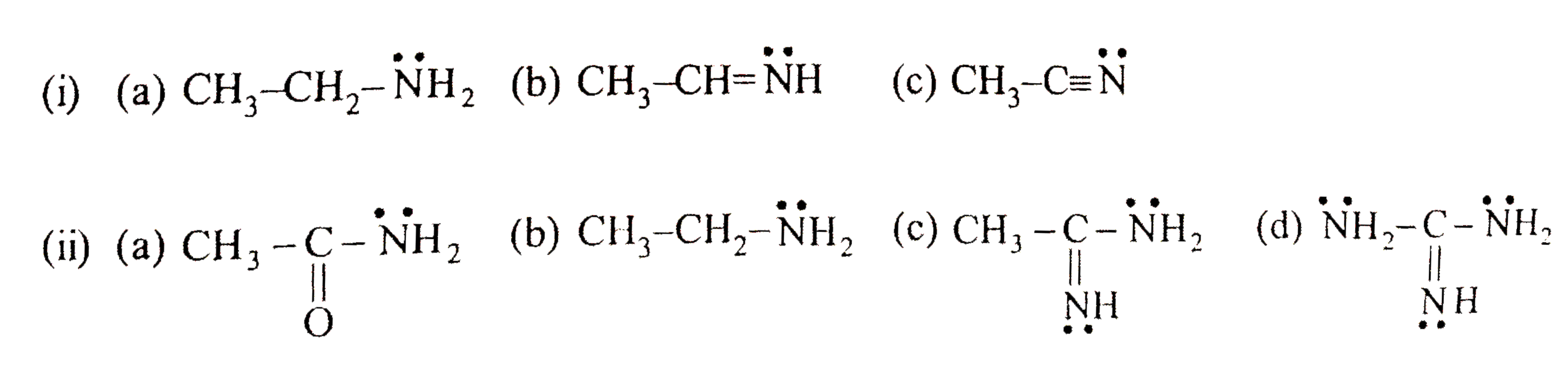What is the increasing order of basic strength of the following compounds in aqueous solution?

A
III<I<II<IV
B
I<II<IV<III
C
I<II<III<IV
D
III<I<IV<II
Video Solution
Text Solution
The correct Answer is:A
In aqeous solution, amine basically follows the order NH3< tertiary< primary < secondary.
This is due to the combined effect of induction and stabilisation of conjugate acid through H-bonding. Also, a cyclic amine is more basic (IV) than acyclic amine. (II). if their degrees are same. hence, the overall order is Kb:III(3∘)<I(1∘)<II(2∘,acyclic)<IV(2∘,cyclic).
|
Similar Questions
Knowledge Check
Order of basic strength in the following compounds.
 , AII>I>III>IVBI>II>IV>IIICIII>II>I>IVDII>I>IV>III
, AII>I>III>IVBI>II>IV>IIICIII>II>I>IVDII>I>IV>IIIThe correct increasing order of basic strength for the following compounds is
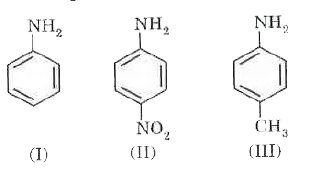 AIII < I < IIBIII < II < ICII < I < IIIDII < III < I
AIII < I < IIBIII < II < ICII < I < IIIDII < III < IThe correct increasing order of basic strength of the following compounds is……
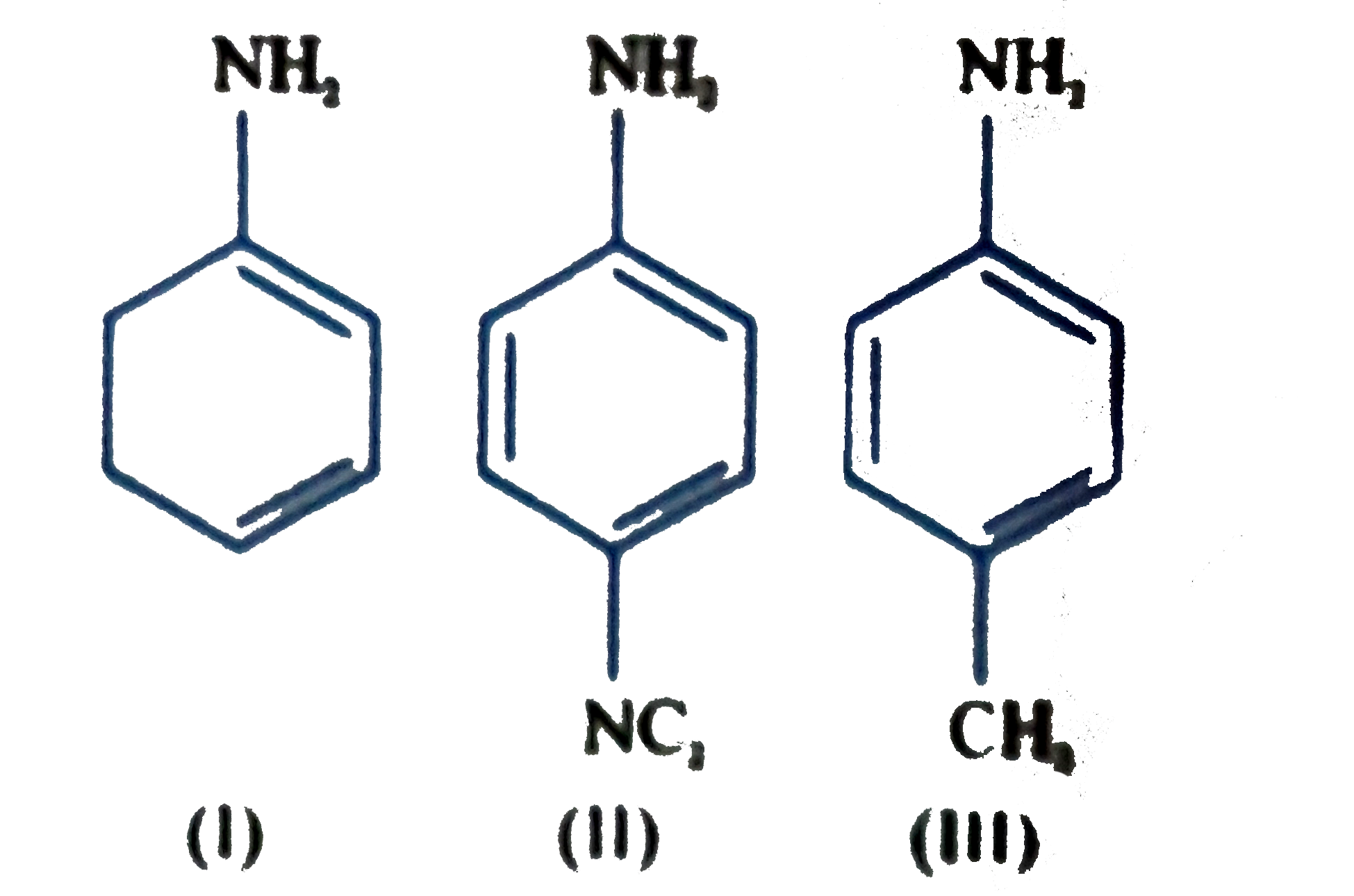 AII<III<IBIII<I<IIICIII<II<IDII<I<III
AII<III<IBIII<I<IIICIII<II<IDII<I<III
The correct increasing order of basic strength for the following compounds is
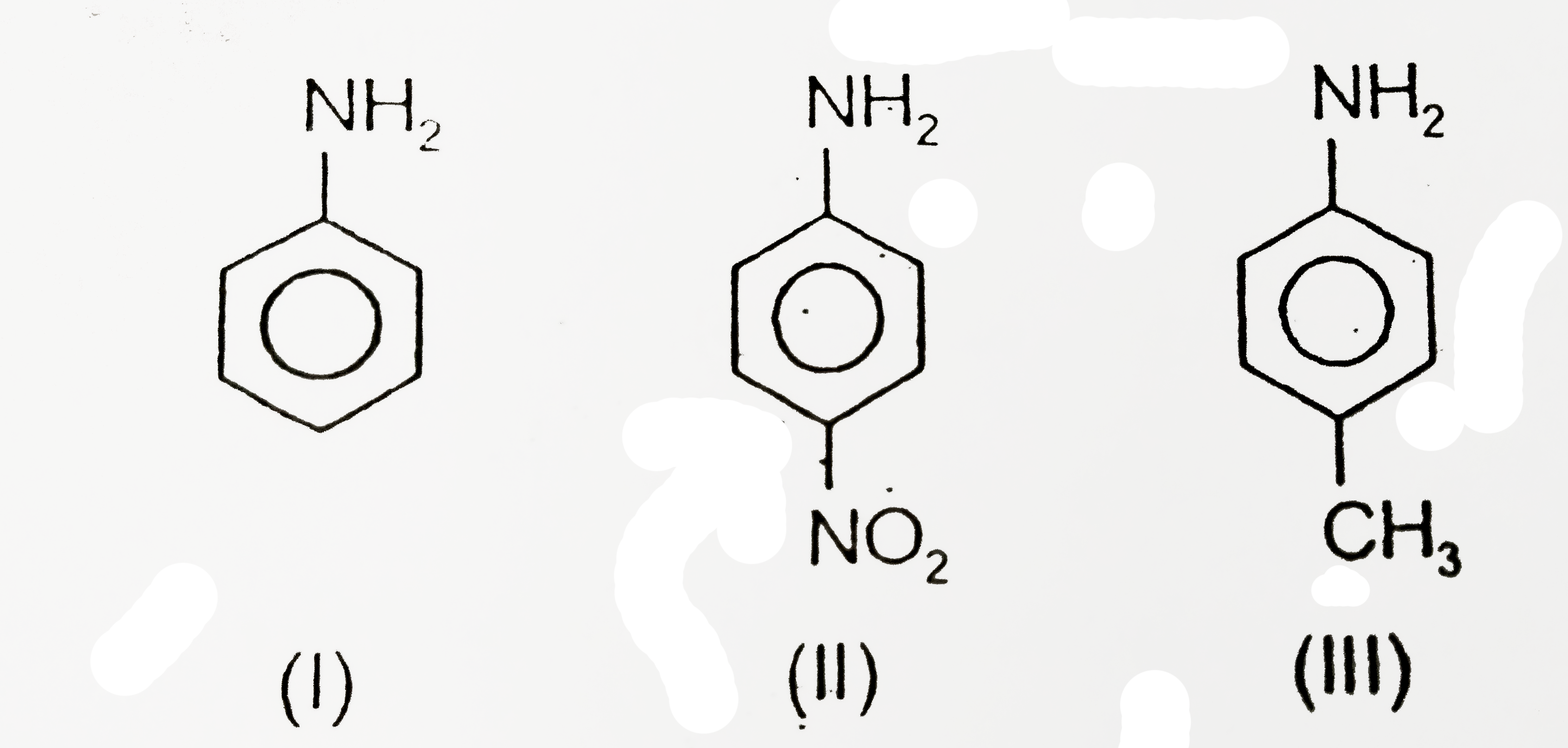
View SolutionThe correct increasing order of basic strength for the following compounds is
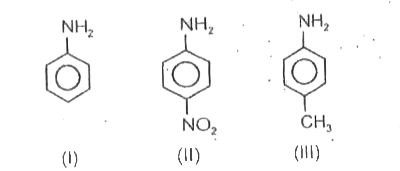
View SolutionThe correct increasing order of basic strength for the following compounds is
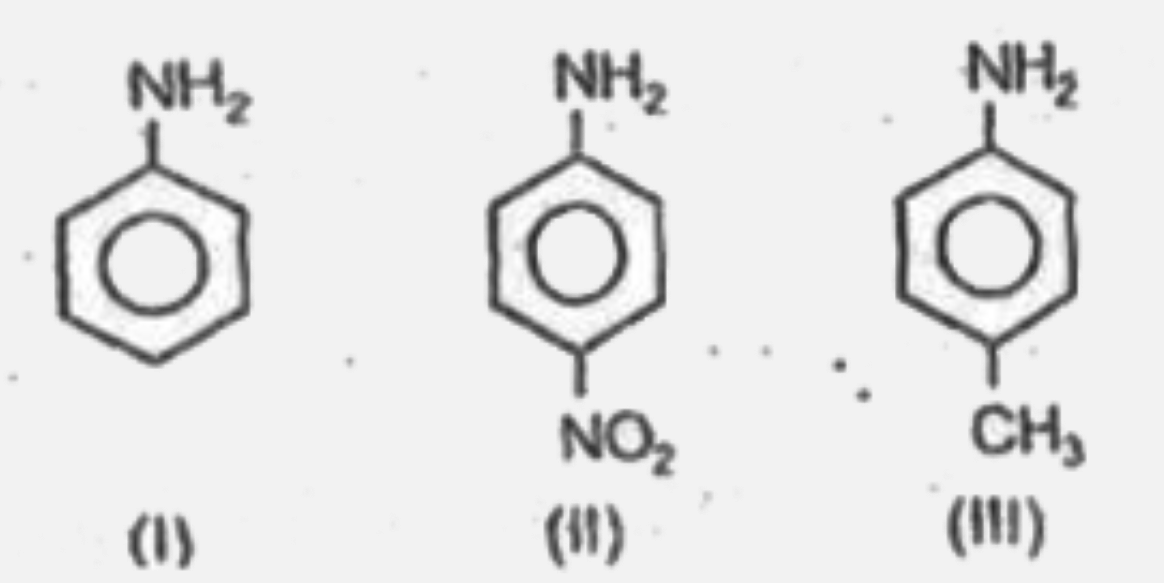
View SolutionThe correct increasing order of basic strength for the following compounds is
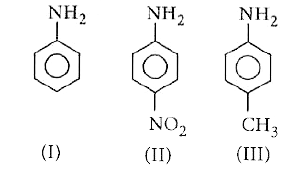
View SolutionThe correct increasing order of basic strength for the following compounds is
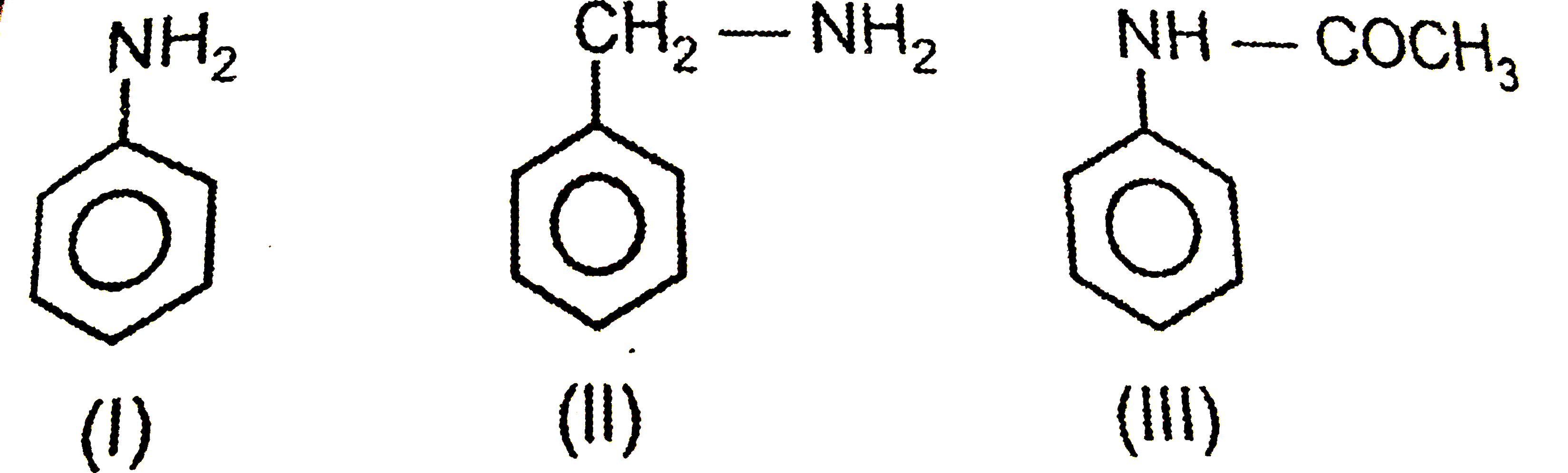
View Solution