The corrent order of dipole moment of alkyl halides is .
A
RI>RBr<RCI>RF
B
RF>RCI>RBr>RI
C
RCI>RF>RBr>RI
D
None of these
Video Solution
Text Solution
The correct Answer is:C
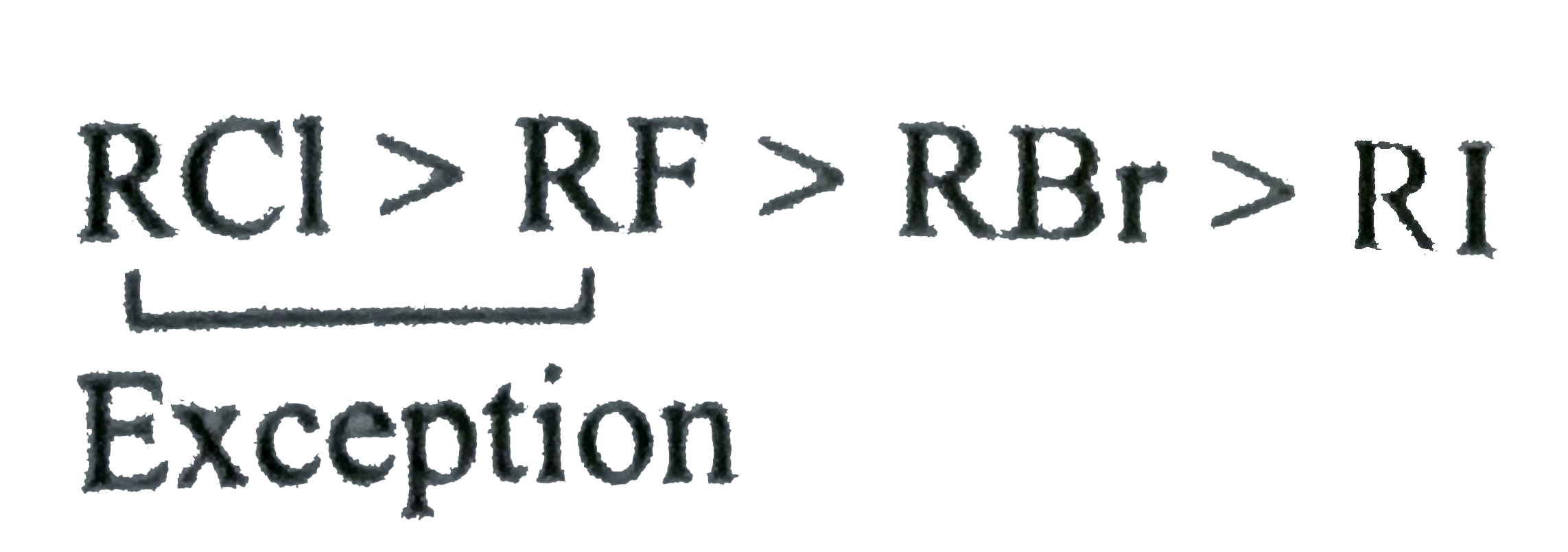
due to bond length polarity oo EN difference .
|
Similar Questions
Knowledge Check
The correc order of dipole moment is
ACH3F<CH3Cl<CH3Br<CH3IBCH3Cl>CH3F>CH3Br>CH3ICCH3F>CH3Cl>CH3Br>CH3IDCH3Cl>CH3F>CH3I>CH3BrThe correct order of dipole moment is
ACH4<NF3<NH3<H2OBNF3<CH4<NH3<H2OCNH3<NF3<CH4<H2ODH2O<NH3<NF3<CH4The correct order of dipole moment is :
ACH4<NF3<NH3<H2OBNF3<CH4<NH3<H2OCNH3<NF3<CH4<H2ODH2O<NH3<NF3<CH4

