The correct order of dipole moment is :
A
PCl3F2>PF3Cl2
B

C
CH3F<CH3Cl
D
SO2<SO3
Text Solution
The correct Answer is:C
μSO3=0,μSO2≠0
|
Similar Questions
Knowledge Check
The correct order of dipole moment is :
ACH4<NF3<NH3<H2OBNF3<CH4<NH3<H2OCNH3<NF3<CH4<H2ODH2O<NH3<NF3<CH4The correct order of dipole moment is :
ACH4<NF3<NH3<H2OBNF3<CH4<NH3<H2OCNH3<NF3<CH4<H2ODH2O<NH3<NF3<CH4The correc order of dipole moment is
ACH3F<CH3Cl<CH3Br<CH3IBCH3Cl>CH3F>CH3Br>CH3ICCH3F>CH3Cl>CH3Br>CH3IDCH3Cl>CH3F>CH3I>CH3Br
Correct order od dipole moment is :
View SolutionIdentify the correct order of dipole moment in the following:
View SolutionThe correct order of dipole moment for the following molecules is
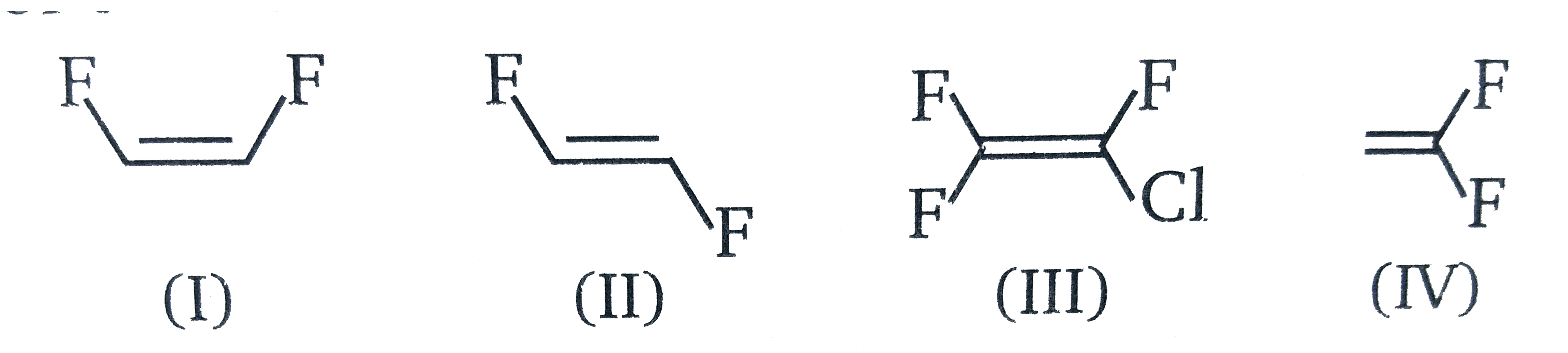
View SolutionThe correct order of dipole moment for the following molecules is
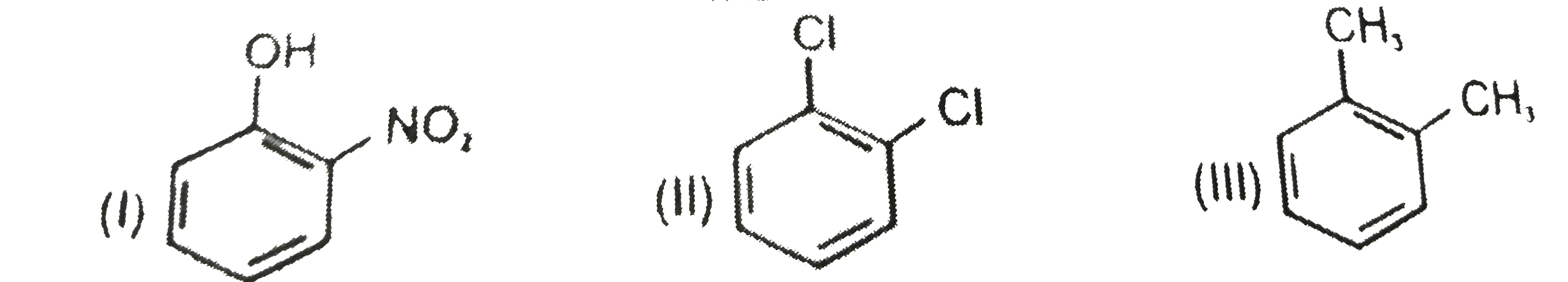
View SolutionThe correct order of dipole moment for the following molecules is
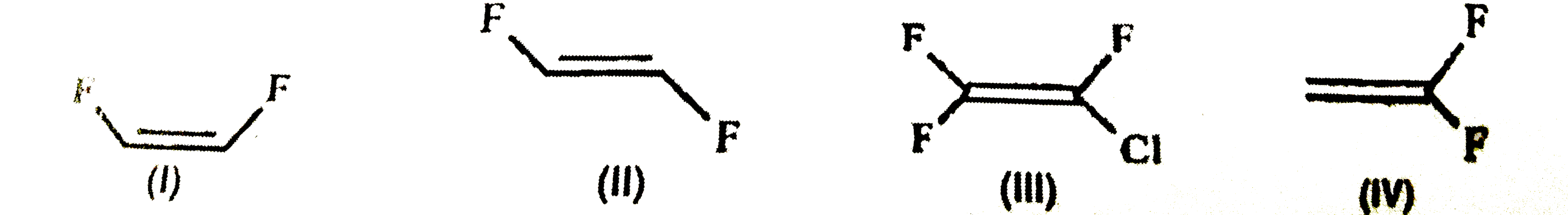
View Solution

