E1 cB reaction is gives by which of the following
CF3−CHCI2
C6H5−C∣NO2H−CH2Br
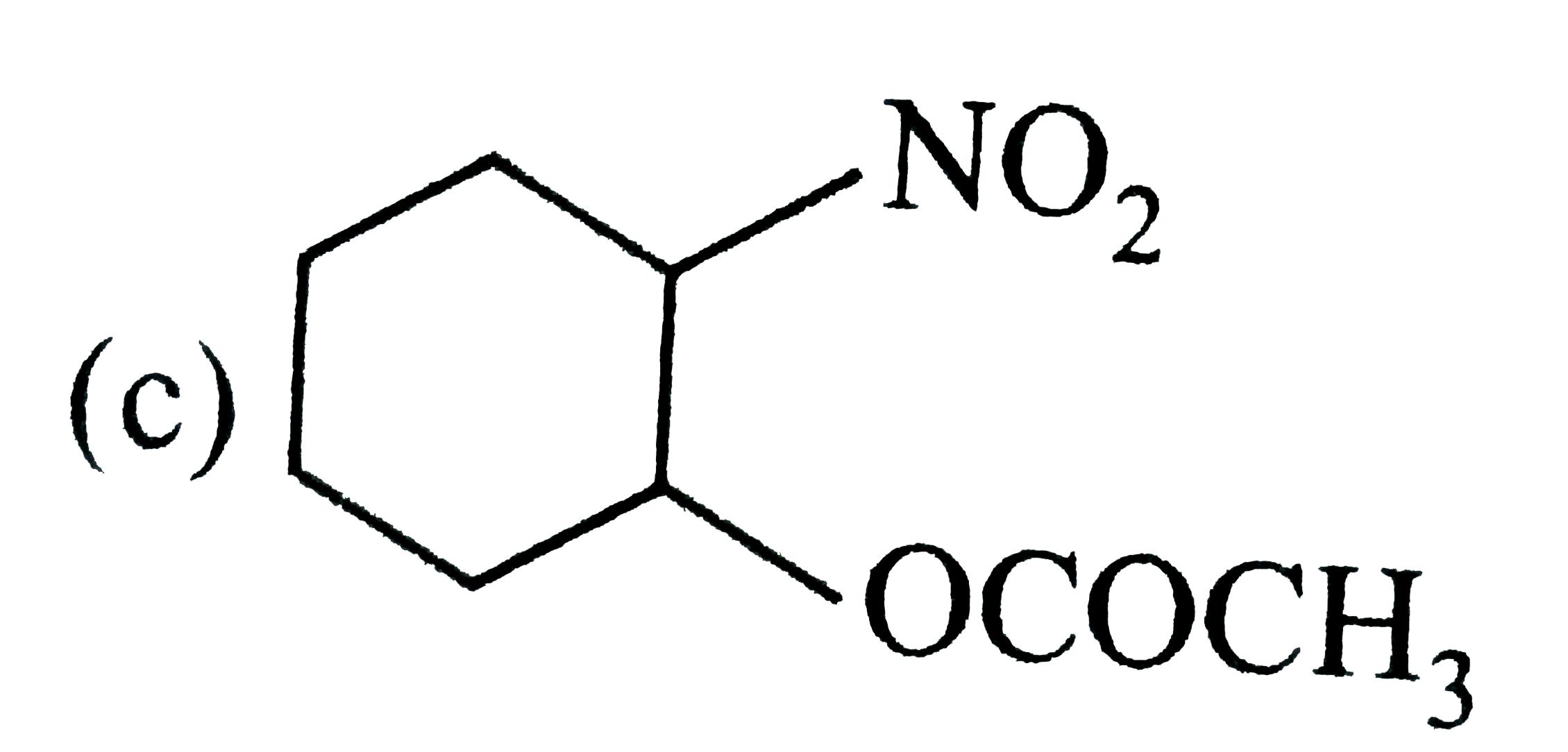 .
.
All of these
The correct Answer is:D
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINING HALOGENSBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING HALOGENSExercise:Methods Of Preparationof Haloarenes
Explore 19 Videos
- ORGANIC COMPOUNDS CONTAINING HALOGENSBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING HALOGENSExercise:Chemical Properties Of Haloarenes
Explore 31 Videos
- ORGANIC COMPOUNDS CONTAINING HALOGENSBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING HALOGENSExercise:Physical Properties And Nuleophilic Substitution Reaction
Explore 34 Videos
- MOCK TESTBook:A2ZChapter:MOCK TESTExercise:Mock Test 2
Explore 45 Videos
- ORGANIC COMPOUNDS CONTAINING NITROGENBook:A2ZChapter:ORGANIC COMPOUNDS CONTAINING NITROGENExercise:Section D - Chapter End Test
Explore 30 Videos
Similar Questions
Knowledge Check
If the E∘cell for a given reaction has a positive value, then which of the following gives the correct relationship for the values of ΔG∘ and Keq :-
AΔG∘<0,Keq<1BΔG∘>0,Keq<1CΔG∘>0,Keq>1DΔG∘<0,Keq>1If the E∘ for a given reaction has a negative value, then which of the following gives the correct relationship for the of ΔG∘ and kaq?
AΔG∘>0,keq<1BΔG∘>0,keq>1CΔG∘<0,keq>1DΔG∘<0,keq<1.If the E∘cell for a given reaction has a negative value, then which of the following gives the correct relationships for the values of ΔG∘andKeq,
AΔG∘>0,kaq>1BΔG∘<0,Keq>1CΔG∘<0,Keq<1DΔG∘>0,Kaq<1
The elimination reactions mainly involve three mechanism E1,E2 and E1cB. If the leaving group departs before β-proton (Ht=Θ ion) then it is E1 mechanism. If proton is taken off first before leaving group it is E1cB mechanism.The pure E2 involves both β-Hydrogen and leaving group departing simutaneously. If acidity of β-Hydrogen increases and leaving group ability decreases then E1cB mechanism increases.
Which of the following substrate will undergo fastest reaction through E1cB route
View SolutionIf the E∘cell for a given reaction has a positive value, then which of the following gives the correct relationship for the values of ΔG∘ and Keq :-
View SolutionThe activation energies of two reactions are Ea1 and Ea2 with Ea1>Ea2. If the temperature of the reacting systems is increased from T to T', which of the following is correct?
View Solution
Use the E∘ values in the table above to the determining which of the following reactions will give the highest potential in a voltaic cell?
View SolutionIf the E∘cell for a given reaction has a positive value, then which of the following gives the correct relationship for the values of ΔG∘ and Keq :-
View Solution

