SN1 reation is faster in
CH3CH2CI
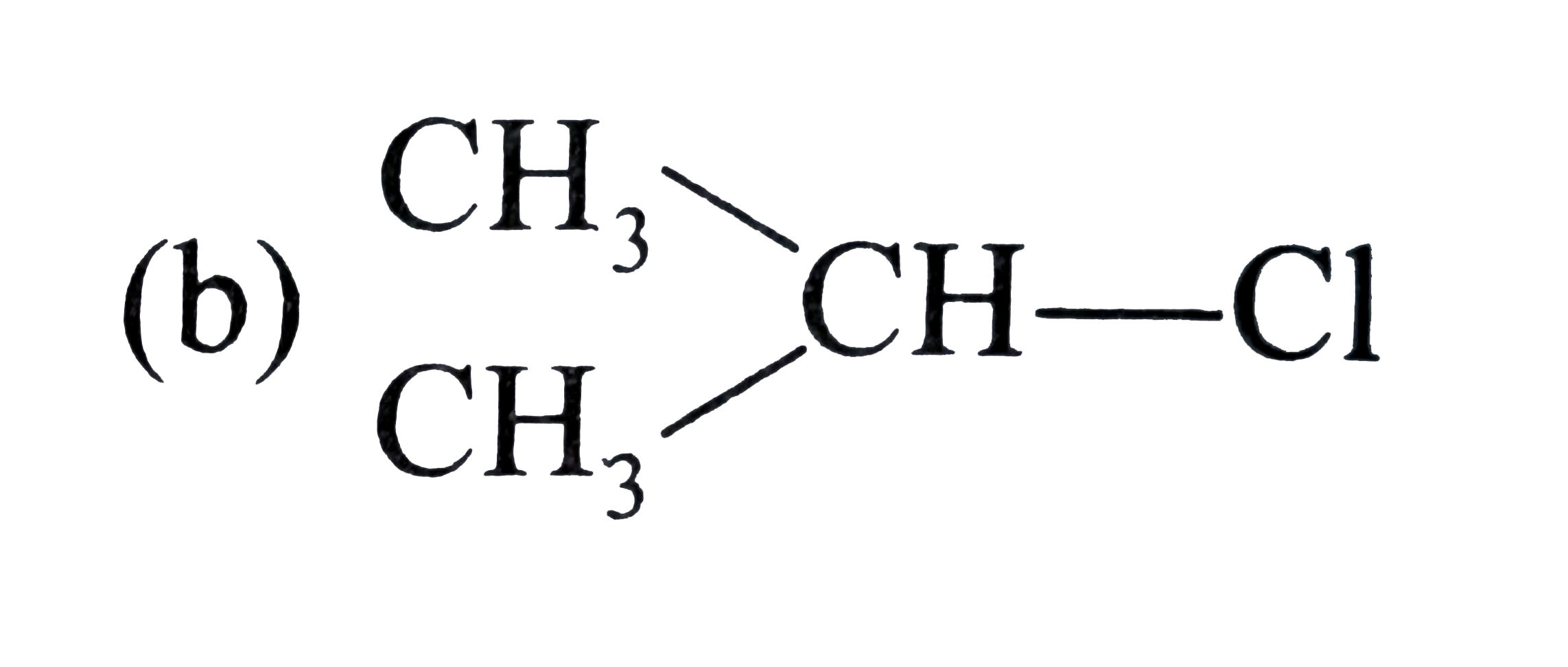
CH3−CH3∣C∣CH3−CI
CH−CH∣CH2∣CH3−CI
The correct Answer is:C
SN1 mechanism proceeds through carbocation intermediate
CH3−CH3∣C∣CH3−CI provides stable carbocation intermediate because it has tertiary carbon .
Similar Questions
Knowledge Check
SN1 reaction is.
Asingle step reationBa reaction involving free readical intermediateCtwo step reactionDtwo step and reaction intermediate is carbocationSN1 reaction is fastest in
ACH3CH2BrBCH3−CH∣Cl−CH3CCH3−CH3C∣∣CH3−ClDCH3−CH∣CH2∣CH3−ClSN1 reaction is feasibole in-
A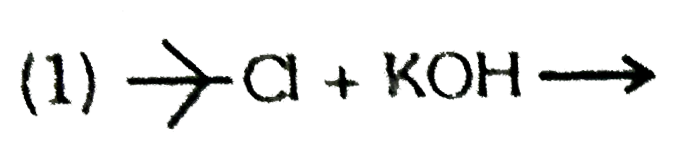 B
B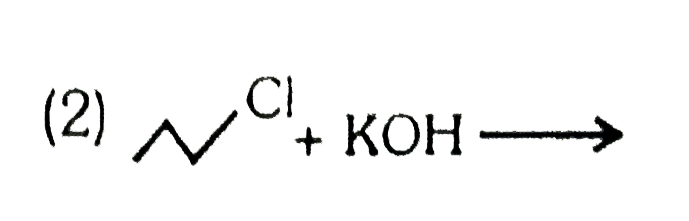 C
C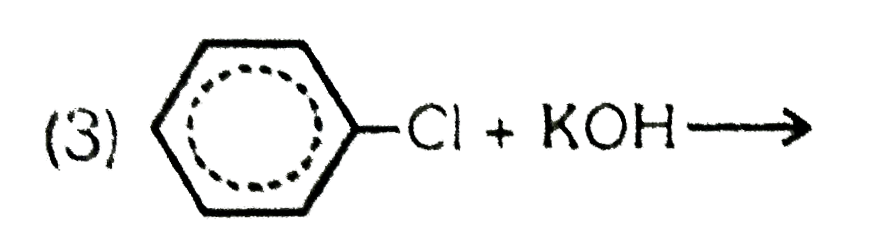 D
D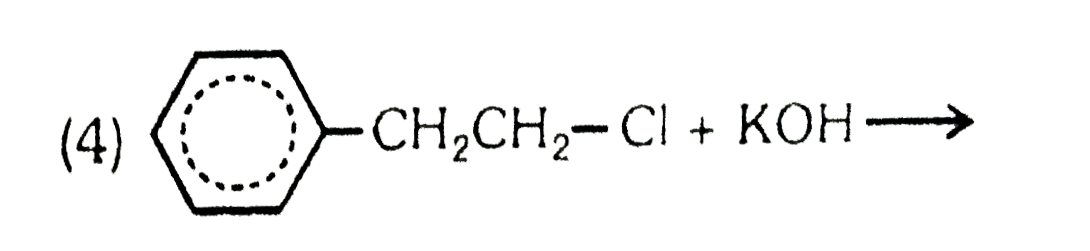
Which of the following compounds would undergo SN1 reaction faster and why ?
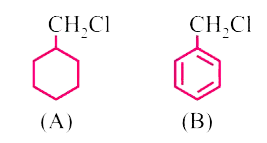
View SolutionSN1 Reaction
View SolutionIn an SN1 reation on chiral centers there is .
View SolutionNucleophilic substitution reactions are of two types, substitution nucleophllic bimolecular (SN2) and substitution nucleophilic unimolecular (SN1) depending on molecules taking part in determining the rate of reaction. Reactivity of alkyl halide towards SN1 and SN2 reactions depends on various factors such as steric hindrance, stability of intermediate or transition state and polarity of solvent. SN2 reaction mechanism is favoured mostly by primary alkyl halide then secondary and then tertiary. This order is reversed in case of SN1 reactions.
In the following pairs of halogen compounds, which would undergo SN2 reaction faster.
View SolutionNucleophilic substitution reactions are of two types, substitution nucleophllic bimolecular (SN2) and substitution nucleophilic unimolecular (SN1) depending on molecules taking part in determining the rate of reaction. Reactivity of alkyl halide towards SN1 and SN2 reactions depends on various factors such as steric hindrance, stability of intermediate or transition state and polarity of solvent. SN2 reaction mechanism is favoured mostly by primary alkyl halide then secondary and then tertiary. This order is reversed in case of SN1 reactions.
In the following pairs of halogen compounds, which would undergo SN2 reaction faster.
View Solution


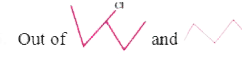 which undergo
which undergo