Which statement is incorrect about free radical halogenation of alkanes ?
A
The number of product molecules formed by one photon is very high.
B
If O2 is added , initially the rate of reaction decreases, then increases.
C
Inhibitors combine with free radical and terminate the chain reaction.
D
Presence of C6H5−C∣∣OO−OC∣∣O−C6H5 inhibits the free radical reaction.
Video Solution
Text Solution
The correct Answer is:D
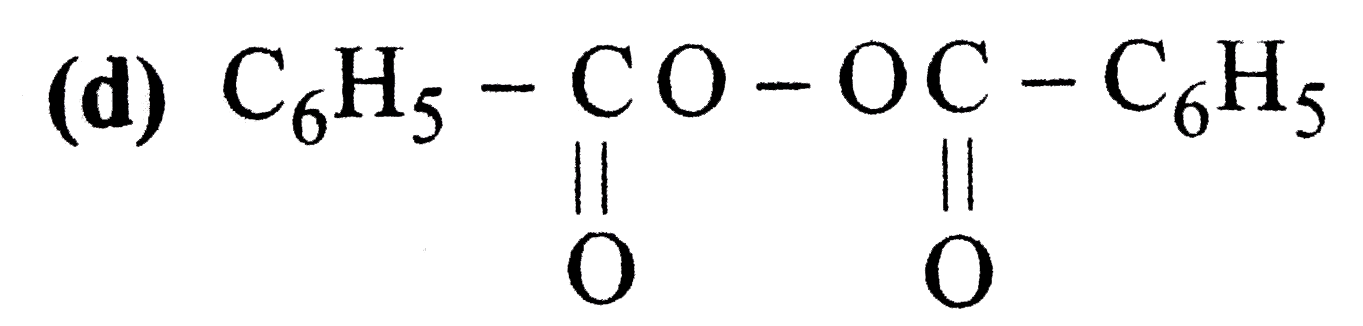 is an initiator in free radical raction.
is an initiator in free radical raction.
|
Similar Questions
Knowledge Check
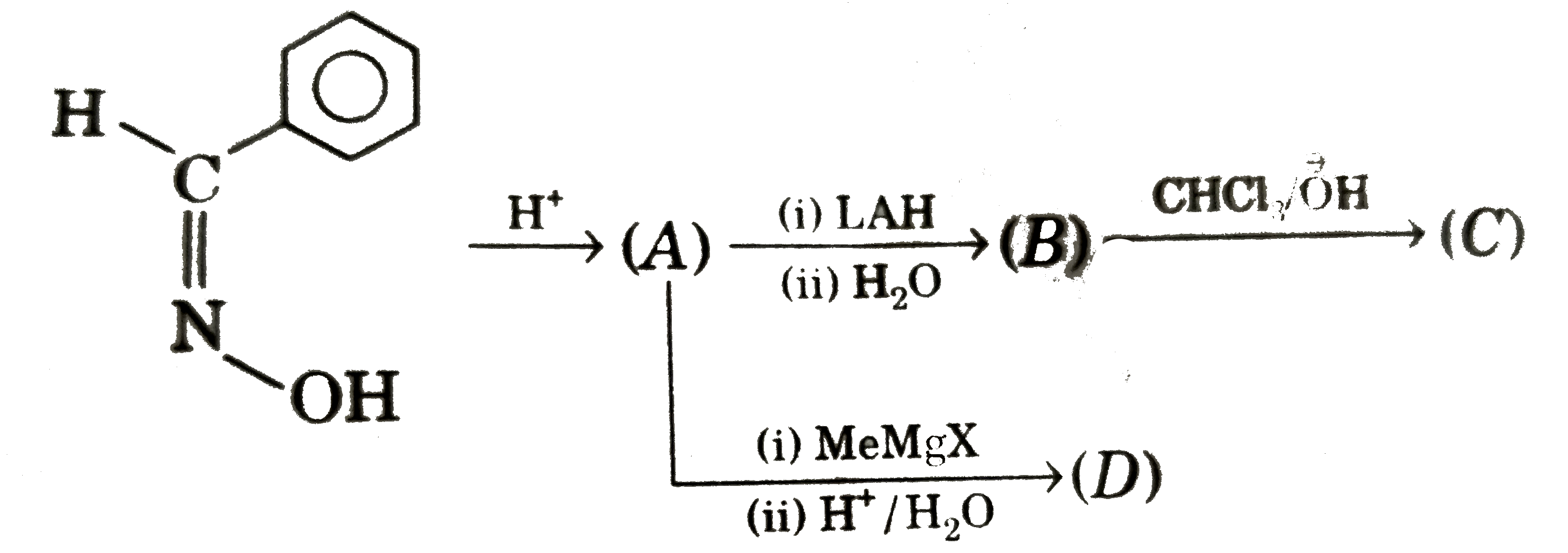
Which statement is incorrect about D?AD can produce yellow ppt. on reaction with Θ OH/I2BD can be prepared by Ph−C≡CH on reaction with Hg2+/H+/H2OCD can form geometrically isomeric oximes on reaction with NH2−OHDD can be converted into alcohol on reaction with Zn(Hg)/HCl.Halogenation of alkanes is
AA reductive processBAn oxidative processCAn isothermal processDAn endothermal processHalogenation of alkanes is
AA reductive processBAn oxidative processCAn isoathermal processDAn edothermic process
