The product obtained by treating
CH3−CH=CH2+HBr→?
A
CH3−CH2−CH2Br
B
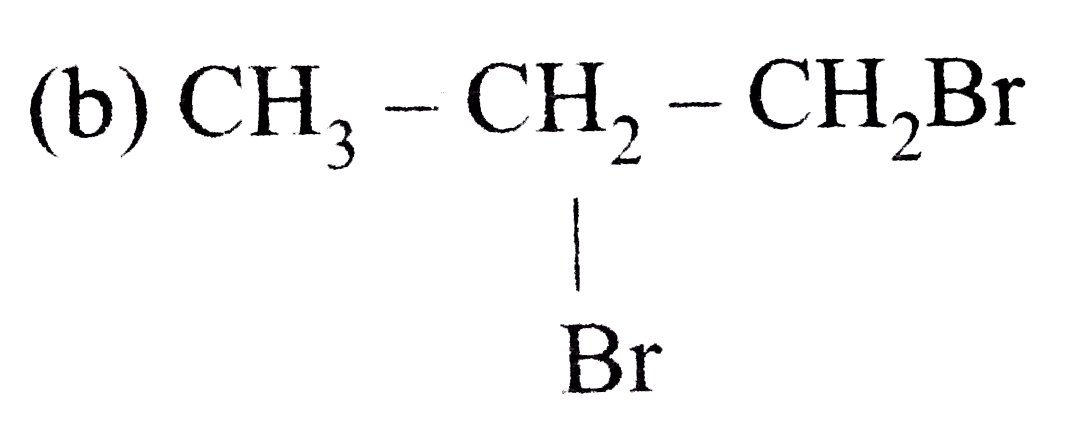
C
CH2BrCH=CH2
D
CH3−CH=CHBr
Text Solution
The correct Answer is:b
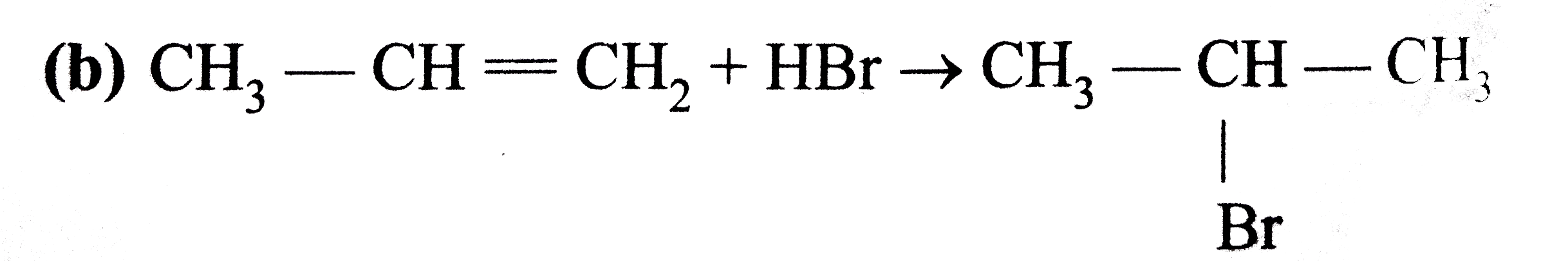
Here Markownikoff's rule is followed which states that negative part of addendum goes to that carbon which has least hydrogen.
|
Topper's Solved these Questions
- HYDROCARBONSBook:A2ZChapter:HYDROCARBONSExercise:Assertion-Reasoning Questions
Explore 5 Videos
- HYDROCARBONSBook:A2ZChapter:HYDROCARBONSExercise:Section D - Chapter End Test
Explore 30 Videos
- HYDROCARBONSBook:A2ZChapter:HYDROCARBONSExercise:AIPMT/NEET Questions
Explore 36 Videos
- ENVIRONMENTAL CHEMISTRYBook:A2ZChapter:ENVIRONMENTAL CHEMISTRYExercise:Section D - Chapter End Test
Explore 30 Videos
- HYDROGENBook:A2ZChapter:HYDROGENExercise:Section D - Chapter End Test
Explore 30 Videos
Similar Questions
Knowledge Check
What will be the product of the following reaction?
CH3−CH=CH2+HBr(C6H5CO)2O2−−−−−−−−−→ProductACH3CH2CH2BrBCH3CH(Br)CH3CCH3CBr2CH3DCH3−C∣H=CH2The major product obtained on treatment of CH3CH2CH(F)CH3 with CH3O−/CH3OH is :
ACH3CH2CH(OCH3)CH3BCH3CH=CHCH3CCH3CH2CH=CH2DCH3CH2CH2CH2OCH3The major product obtained on treatment of CH3CH2CH(F)
CH3withCH3O−/CH3OH is .ACH3CH2CH(OCH3)CH3BCH3CH=CHCH3CCH3CH2CH=CH2DCH3CH2CH2CH2OCH3
Complete the reaction :
CH3−CH2−CH2−O−CH3−HBr→?
View SolutionGive the product of the following
(i) C6H5CH=CH2+HBr→
CH3CH2CH=CH2+HCl→
(iii) C6H5CH2CH=CH2+HBrPeroxide−−−−→
View SolutionPredict the products of the following reactions:
(i) CH3−CH2−CH2−O−CH3+HBr→
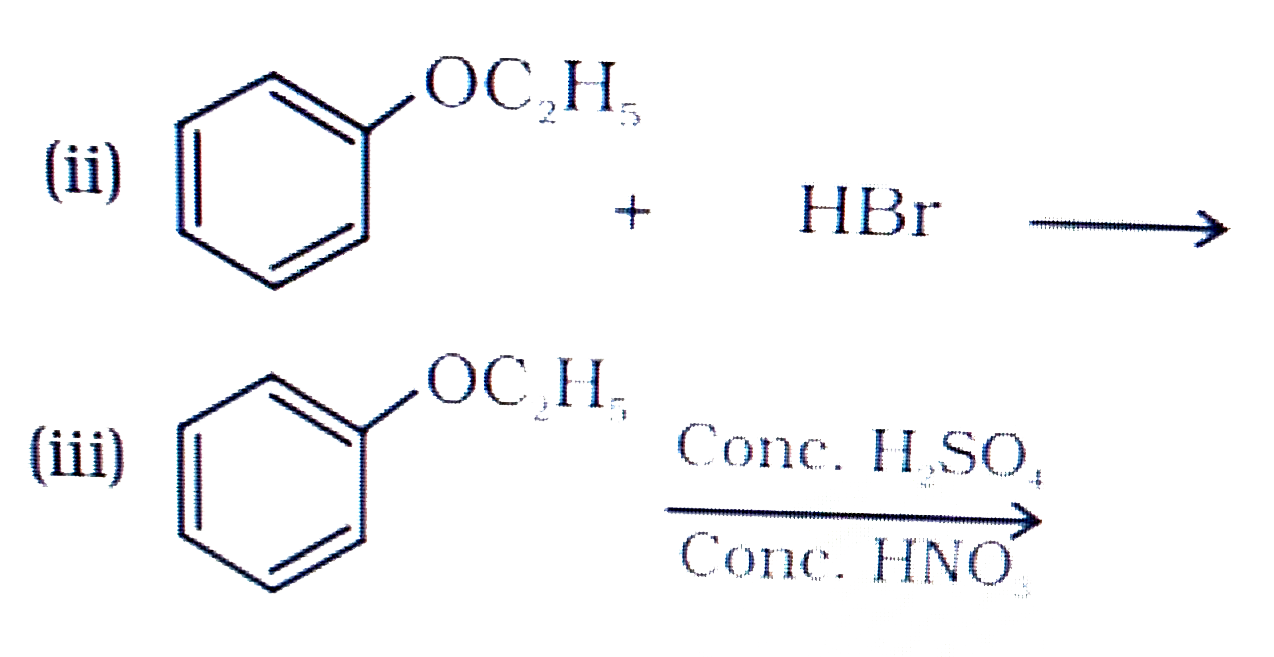
(iv) (CH3)3C−OC2H5HI−−→
View SolutionThe major product obtained on treatment of CH3CH2CH(F)CH3 with CH3O−/CH3OH is :
View SolutionThe product of the following reaction:
CH2=Ch−CCl3+HBr→is ________.
View Solution

