Two isomeric compounds A and B have molecular formula C3H6O. A responds to silver mirror test but not B. B on treatment with Zn ( Hg) and conc. HCl gives propane. A and B are respectively.
ethanal and propanone
propanone and ethanal
propanal and propanone
propanaone and propanal
The correct Answer is:C
C3H6O⇒ Carbonyl compound.
+ve silver mirror test ⇒ A is propanal.
Bundergoes Clemmensen's reaction ⇒ B is acetone.
Similar Questions
Two isomeric compounds A and B having molecular formula C4H11N, both lose N2 on treatment with HNO2 and gives compound C and D, respectively. C is resistant to oxidation but immediately responds to Lucas reagent, whereas 'D' responds to Lucas reagent after 5 minutes and gives a positive iodoform test. Identify A and B.
View SolutionTwo isomeric aromatic compounds A and B have the molecular formula C7H7OH. A gives purple colour with FeCl3 solution while B does not. What are A and B ?
View Solution
Knowledge Check
Two isomeric compounds A and B have some molecualr formula C3H5N. It was found that
AA and B do not react with NHO2 or CH3COClBOn refluxing with dil. HCl, A gives C and B gives D. Both C and D are monobase carboxylic acids.CMolecular mass of D is 74.DC gives positive Tollen's test whereas D does not predict the structures of A,B,C and D.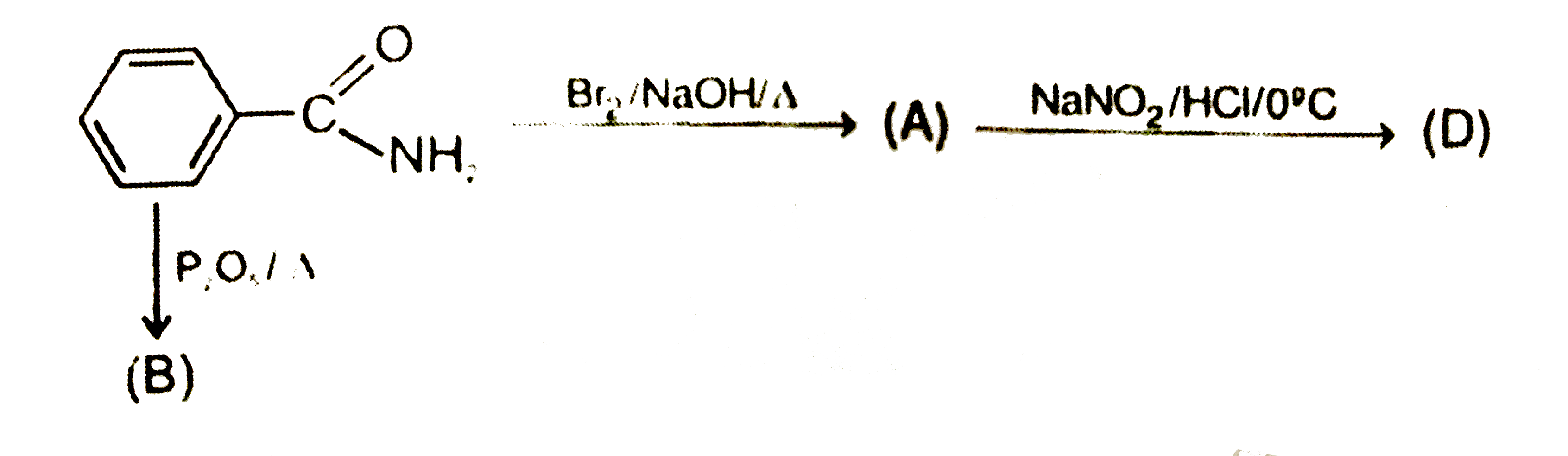
(B) on treatment with Zn/HCl gives :AanilineBbenzylamineCphenylisocyanideDN-methylanilineA organic compound with the molecular formula C3H6O does not respond positively to the silver mirror test with Tollens reagent but produces on oxmime. The compound is
ACH2=CHCH2OHBCH3CH2CHOCCH2=CHOCH3DCH3COCH3
How many of the osomeric ketones having the molecular formula C6H12O undergo iodoform test?
View Solution(a) An organic compound 'A' having molecular formula C4H8 on treatment with dil. H2SO4 gives B. B on treatment with conc. HCI and anhydrous ZnCI2 gives secondary halide C. Write all the reactions and identify A, B and C.
(b) Convert ethyl chloride into methyl chloride.
View SolutionTwo isomeric compounds(A) and (B) have molecular formula C6H10. Oxidation of (A) gives mixture of butanoic and acetic acids, whereas compound (B) gives hexa-1,6-dioicacid. What are (A) and (B)?
View SolutionAn organic compound 'A' has the molecular formula C3H6O, it undergoes iodoform test. Whet satruated with HCl it gives 'B' of molecular foumula C9H14O. A and B, respectively are
View Solutionwhich of the structural isomeric ketones having molecular formula (C5H10O) give iodoform test ?
View Solution

