Carboxylic acids are acidic in nature because :
A
it dissociates to give H+ ioins
B
it dissolves in water to form protons
C
the carboxylate ion is resonance unstablised
D
both (a) and (b)
Video Solution
Text Solution
The correct Answer is:D
R−COOH→R−COO−+H+
R−COOHH2O−−→R−COO−+H+
Acidic nature of RCOOH.
|
Similar Questions
Knowledge Check
Carboxylic acid can
Adecompose carbonate and evolve CO2Breacts with metal forming H2 gasCneutralise ammonium hydroxide form saltDall of the above
Carboxylic acid (A) is:A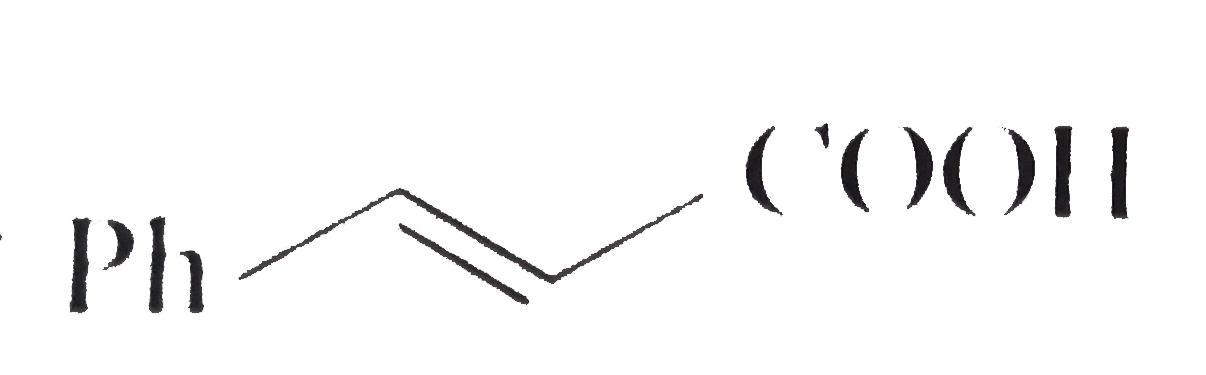 B
B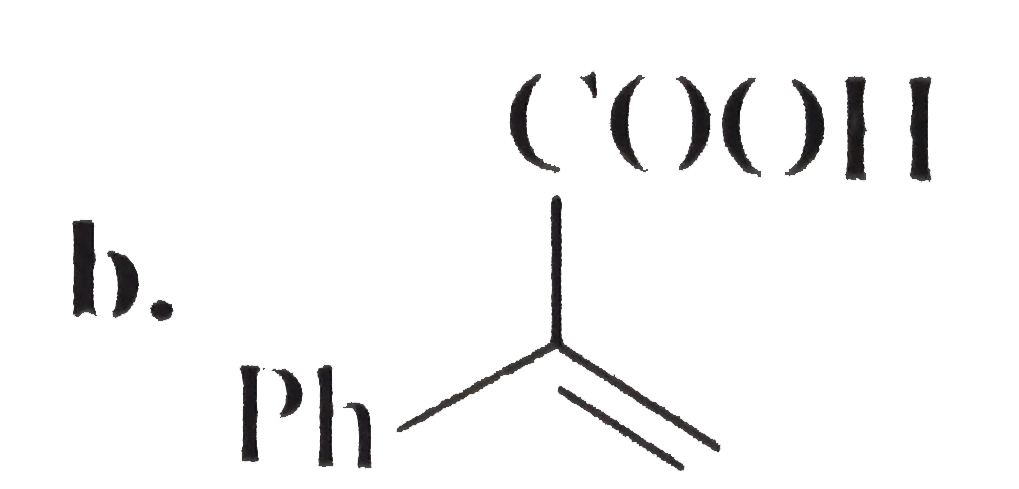 C
C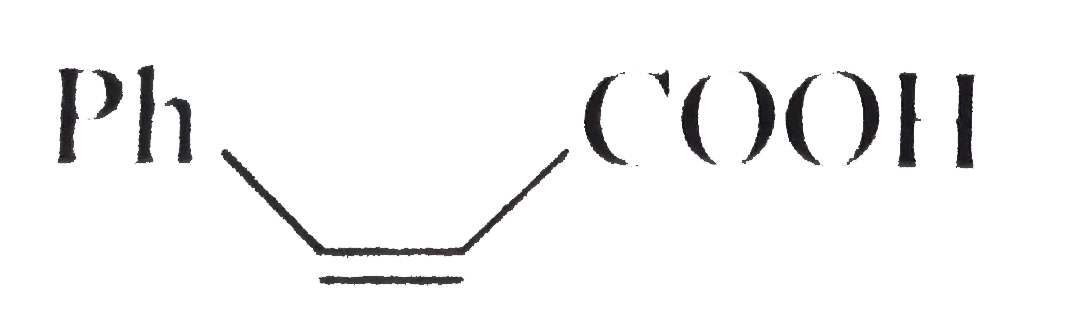 DBoth (a) and (c )
DBoth (a) and (c )Carboxylic acids are more acidic than phenol and alcohol because of
Aintermolecular hydrogen bondingBformation of dimersChighly acidic hydrogenDresonance stabilization of their conjugate base
Carboxylic acid is isomeric with
View SolutionCarboxylic acid on reduction produces
View SolutionCarboxylic acids are converted into alkanes by
View SolutionWhich of the following carboxylic acids is most acidic in character-
View SolutionWhich of the following structure of carboxylic acid accounts for the acidic nature
View Solution
