.Arrange the following in increasing order of ionization enthalpy: B,C,N,O
Video Solution
|
Answer
Step by step video & image solution for .Arrange the following in increasing order of ionization enthalpy: B,C,N,O by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
Knowledge Check
Arrange the following in increasing order of stability :
AN2<N+2<N−2BN+2<N−2<N2CN−2<N+2<N2DN2<N−<N+2Arrange the following alkanes in increaseing order of their enthalpy
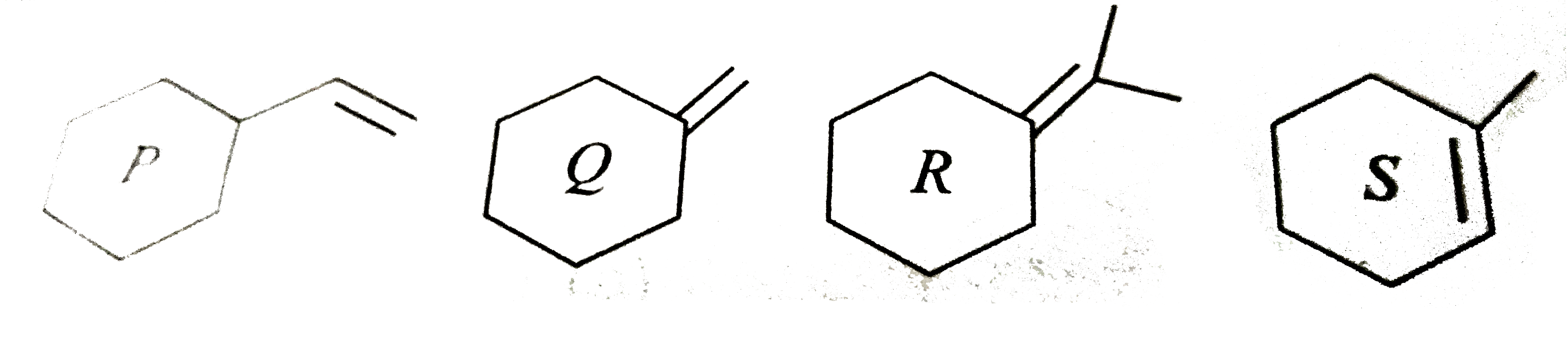 AR<S<Q<PBR<S<P<QCP<Q<R<sDP<Q<S<R
AR<S<Q<PBR<S<P<QCP<Q<R<sDP<Q<S<RArrange the following in the increasing order of their bond order:
AO2,O+2,O−2andO2−2BO2−2,O−2,O2,O+2CO+2,O2,O−2,O2−2DO2,O+2,O−2,O2−2
Arrange the following in order of increasing O.N of iodine:
View SolutionArrange the following in the increasing order of bond length : O2,O−2,O+2
View SolutionArrange the following in the increasing order of acidic strength : phenol, ethanol, o-nitrophenol
View SolutionArrange the following acids in the correct increasing order of their acidic strength,
HCl, HBr, HI, HF
View SolutionArrange the following in the order of increasing ionization potential
View Solution