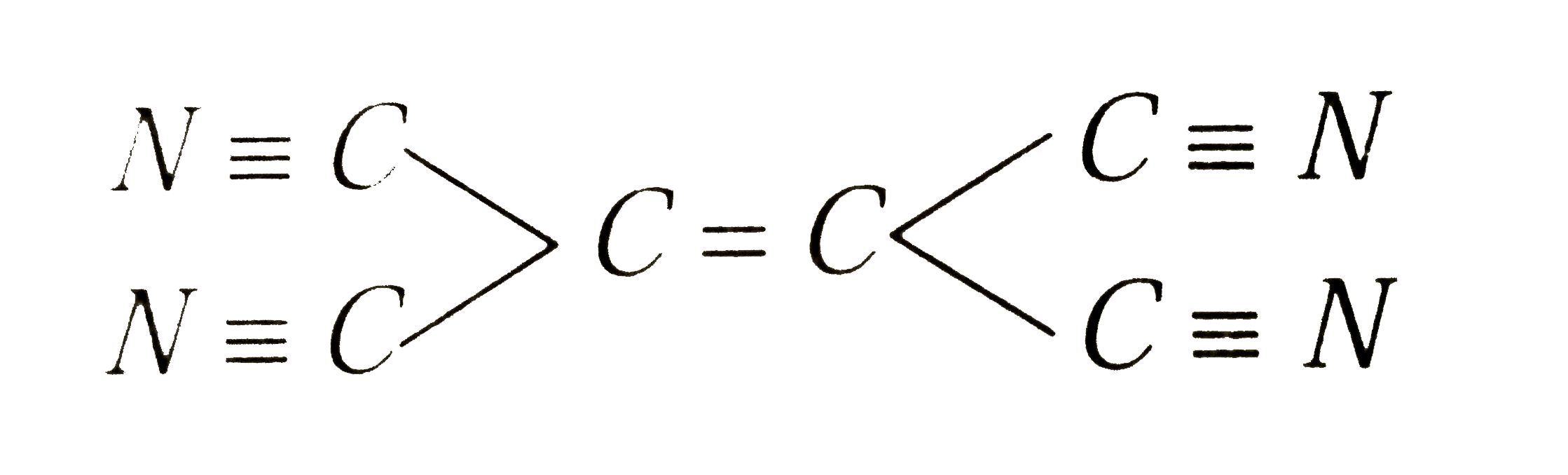The number of sigma (σ) and pi(π) bonds present in a molecule of tetracyanoethene is
A
9σ and 9π
B
9σ and 7π
C
5σ and 9π
D
5σ and 8π
Video Solution
Text Solution
The correct Answer is:A
Draw the structure of tetracyanoethene. It is derived from ethene (CH2=CH2) molecule by replacing all the 4H atoms by cyanide groups (−C≡N):
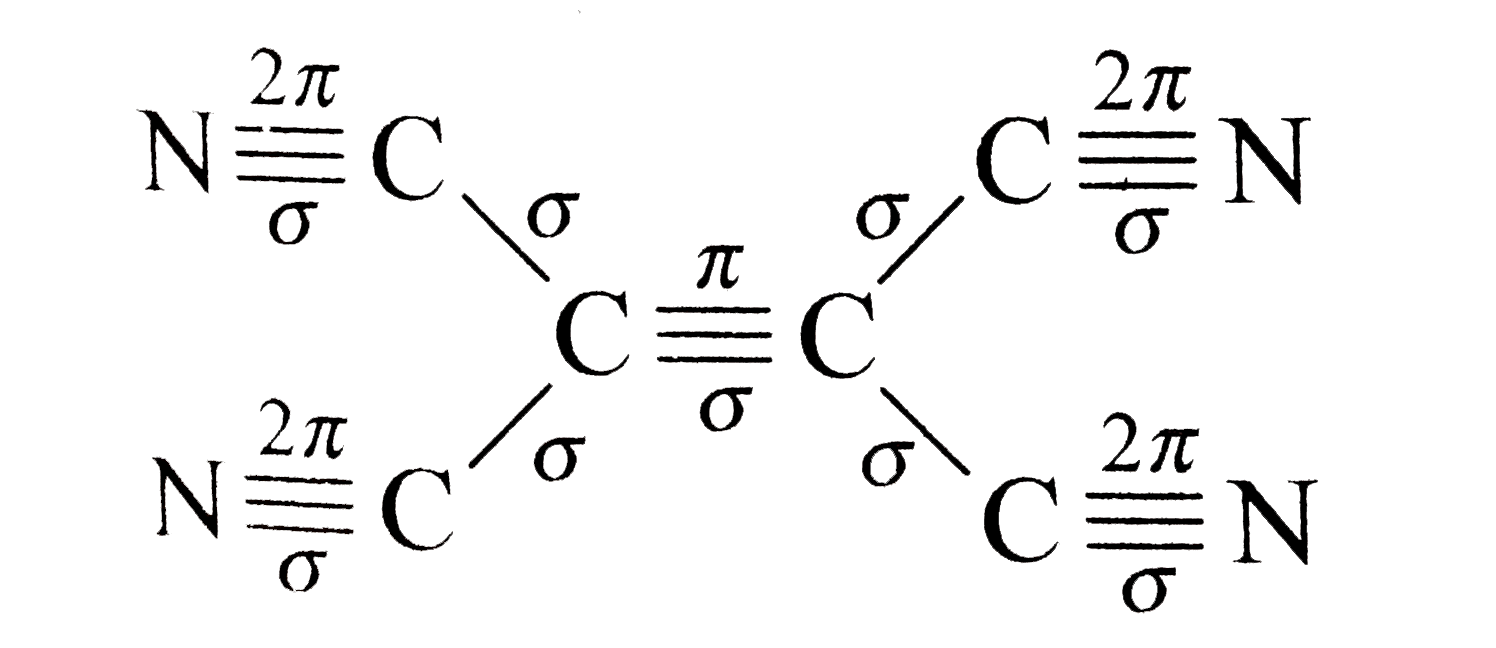
Every single bond is a sigma bond, a double bond contains one sigma and one π bond, and every triple bond contains one sigma and two pi bonds.
|
Similar Questions
Knowledge Check
The number of sigma (σ) and pi (π) bonds in pent-1-en-4-yne is
A10 σ bonds are 3π bondsB8 σ bonds and 5π bondsC11 σ bonds and 2π bondsD13σ bonds and no π bondsThe number of sigma (σ) and pi(π) bonds in pent-2-en-4-yne is:
A10 σ bonds are 3π bondsB8 σ bonds and 5π bondsC11 σ bonds and 2π bondsD13σ bonds and no π bondsThe number of sigma (σ) and pi(π) bonds in pent-2-en-4-yne is:
A13σ bonds and no π bondB10σ bonds and 3π bondC8σ bonds and 5π bondD11σ bonds and 2π bond
How many σ and π bonds are present in the molecule of ethene ?
View SolutionHow many σ-and-πbonds are there in the molecule of tetracyano ethylene?
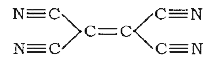
View SolutionHow many sigma and pi bonds are there in the molecule of tetracyano ethylene ? .
View SolutionWhat is the number of sigma (σ) and pi (π) bonds present in sulphuric acid molecule ?
View SolutionThe number of sigma (σ) and pi (π) bonds present in acetylene are _____ respectively.
View Solution