What is the entropy change (in JK−1mol−1) when one mole of ice is converted into water at 0∘C?
(The enthalpy change for the conversion of ice to liquid water is 6.0KJmol−1 at 0∘C)
20.13
2.013
2.198
21.98
The correct Answer is:D
H2O(s)⇔H2O(l)
According to thermodynamics,
ΔSfus=ΔHfusTf
=600Jmol−1273K=21.98Jk−1mol−1
Topper's Solved these Questions
Similar Questions
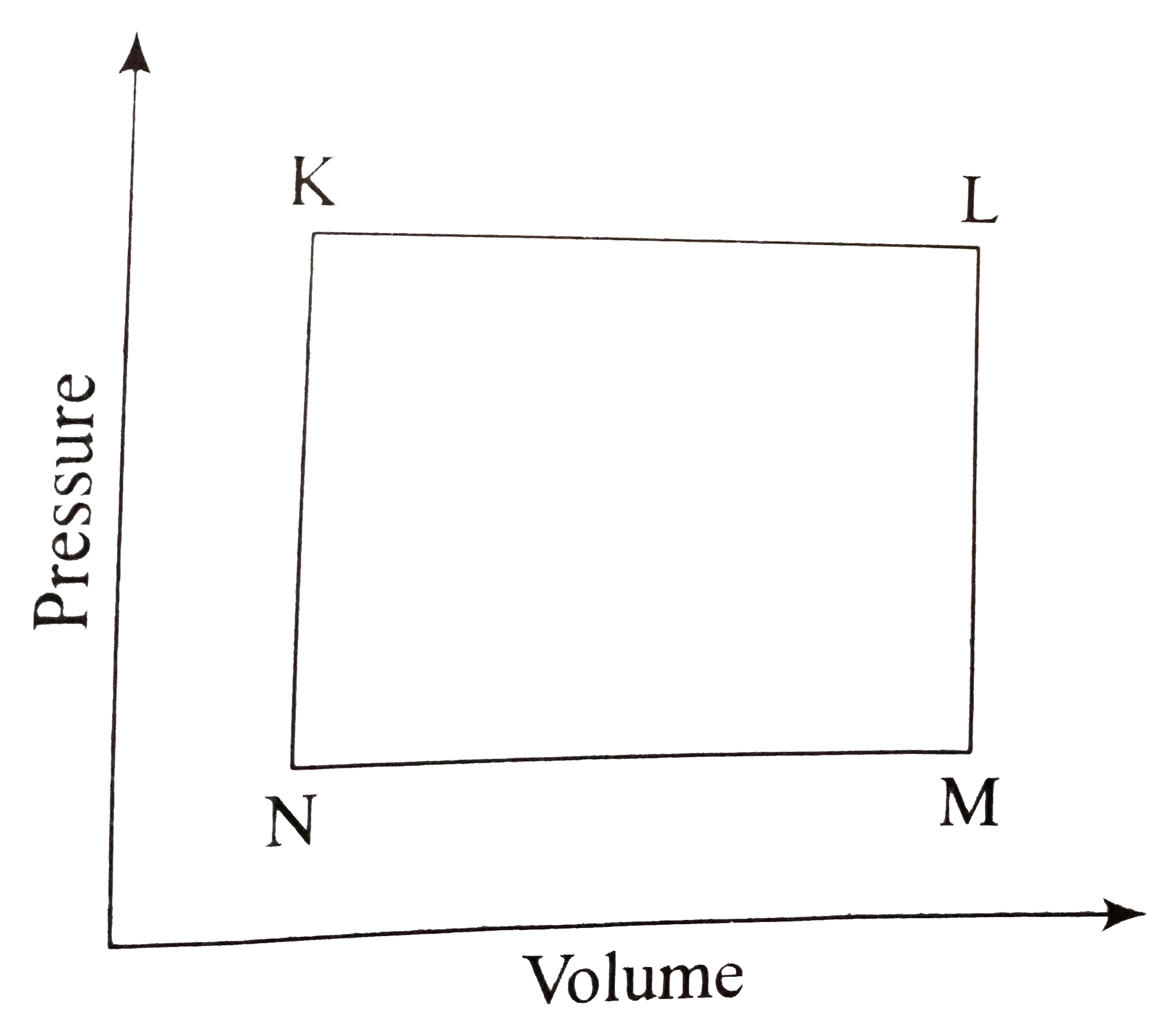
A fixed mass m of a gas is subjected to transformation of state: K to L to M and black to K as shown in the figure.
The succeeding operations that enabel this transformation of state are
View SolutionThe correct order of increasing thermal stability of K2CO3,MgCO3,CaCO3, and BeCO3 is
View SolutionCalculate the entropy change (DeltaS) when 1 mol of ice at 0^(@)C is converted into water at 0^(@)C . Heat of fusion of ice at 0^(@)C is 1436 cal per mol.
View SolutionOne mole of ice is converted into water at 273K . The entropies of H_2O(s) and H_2O(l) are 38.20 and 60.01Jmol^(-1)K^(-1) respectively. The enthalpy change for the conversion is:
View SolutionWhat is the entropy change (in JK^(-1)mol^(-1) ) when one mole of ice is converted into water at 0^(@)C ? (The enthalpy change for the conversion of ice to liquid water is 6.0KJmol^(-1) at 0^(@)C )
View SolutionIf water vapour is assumed to be a perfect gas, molar enthalpy change for vapouration of 1 mole of water at 1 bar and 100^(@)C is 41 kJ mol^(-1) . Calculate the internal energy change when a) 1 mol of water is vapourised at 1 bar and 100^(@)C b) 1 mol of water liquid is converted into ice.
View SolutionCalculate the entropy change during the melting of one mole of ice into water at 0∘C and 1 atm pressure. Enthalpy of fusion of ice is 6008J mol−1 .
View SolutionAt 0∘C and normal pressure, the enthalpy of fusion of ice is 334.7J⋅g−1. At this temperature and pressure, if 1 mol of water iis converted into 1 mol ice, then the change in entropy of the system will be-
View SolutionWhat is the entropy change (in JK^(-1) mol^(-1)) when one mole of ice is converted into water at 0^(@)C ? (The enthalpy change for the conversion of ice to liquid water is 6.0 kJ mol^(-1) at 0^(@)C )
View Solutionजब एक मोल बर्फ को 0∘C ताप पर जल में परिवर्तित किया जाता है, तो एन्ट्रॉपी परिवर्तन (जूल केल्विन मोल' में) होता है (0∘C ताप पर बर्फ से जल बनने पर एन्थैल्पी परिवर्तन 6.0 किलो जूल "मोल"^(-1) है)
View Solution1 मोल बर्फ का परिवर्तन 0∘C पर जल में होता है, एन्ट्रॉपी में परिवर्तन (जूल केल्विन−1 मोल−1) क्या होगा?
[0∘C पर बर्फ के जल में परिवर्तन के लिए एन्थैल्पी परिवर्तन 6.0 किलो जूल मोल−1]
View SolutionCalculate the entropy change during the melting of one mole of ice into water at 0^@C and 1 atm pressure. Enthalpy of fusion of ice is 6008 "J mol"^(-1) .
View SolutionWhat is the entropy change (in JK^(-1)mol^(-1) ) when one mole of ice is converted into water at 0^(@)C ? (The enthalpy change for the conversion of ice to liquid water is 6.0KJmol^(-1) at 0^(@)C )
View SolutionWhat is the entropy change (in JK−1mol−1) when one mole of ice is converted into water at 0∘C?
(The enthalpy change for the conversion of ice to liquid water is 6.0KJmol−1 at 0∘C)
View SolutionCalculate the entropy change (ΔS) when 1mol of ice at 0∘C is converted into water at 0∘C. Heat of fusion of ice at 0∘C is 1436cal per mol.
View SolutionWhat is the entropy change (in JK^(-1) mol^(-1)) when one mole of ice is converted into water at 0^(@)C ? (The enthalpy change for the conversion of ice to liquid water is 6.0 kJ mol^(-1) at 0^(@)C )
View Solution
