Balance the following equation is alkaline medium
Zn(s)=N−3(aq)→Zn2+(aq.)+NH+4(aq).
Strategy : Follow the seven-step procedure, one step at a time.
Video Solution
Text Solution
Step 1: Write the sleletal ionic equation
Zn(s)+NO−3(aq)→Zn2+(aq)+NH+4(aq)
Step 2: Assingn oxidation number to all the atoms :
0Zn(s)++5−2NO−3(aq.)→+2Zn2+(aq.)+−3+1NH+4(aq.)
The oxidation number of Zn increases from 0 to +2 while the oxidation number of N drecease from +5 to −3
Step 3: Idenify oxidizing and reducing agents:
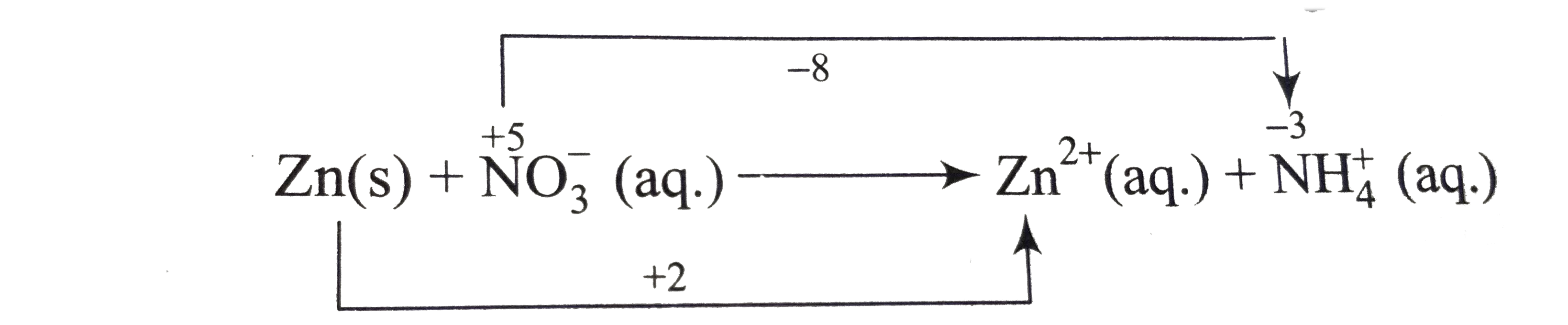 .
.
Zn is the reducing agent as it is oxidized while NO−3 is the oxidizing agent as it is reduced . Since there is only one Zn atom on either side. the total increase in the oxidation number of Zn is of two units. Since there is only one N atom on either side, the total decrease in the oxdation number of N is of eight units.
Step 4: Make the total inceease and decrease in oxidation numbers equal by multiplying Zn (reducing agent ) by 4, Also multiply Zn2+ by 4 to balance Zn atoms . (Step 5)
4Zn(s)0 charge+NO−3(aq.)1- charge→4Zn2+(aq.)4(2+)=8+ charge+NH+4(aq.)1+ charge
Step 6: Balance the ionic charges on both the sides by adding OH− ions as the reaction is carried out in basic condition. On the left side, there is 1− charge due fo NO−3 ion but on the right side, there are 9+ charges due to four Zn2+ ions and one NH+4 ion. Add 10OH− ions on the left to make ionic charges equal
4Zn(s)+NO−3(aq.)→4Zn2+(aq.)+NH+4(aq.)+10OH−(aq.)
Step 7 : Finally balance H and O atoms by adding H2O molecules. There are zero H atoms and three O atoms on the left side. There are 14H atoms and 10O atoms on the left side. Thus, add 7H2O mol- ecules on the left side to achieve the balanced redox reaction : ltbtgt 4Zn(s)+NO−3(aq.)+7H2O→4Zn2+(aq.)+NH+4(aq.)+10OH−(aq).
|
Topper's Solved these Questions
- REDOX REACTIONSBook:R SHARMAChapter:REDOX REACTIONSExercise:Follow-up Test 1
Explore 5 Videos
- REDOX REACTIONSBook:R SHARMAChapter:REDOX REACTIONSExercise:Follow-up Test 2
Explore 5 Videos
- PURIFICATION AND CHARATERIZATION OF ORGANIC COMPOUNDSBook:R SHARMAChapter:PURIFICATION AND CHARATERIZATION OF ORGANIC COMPOUNDSExercise:Archives
Explore 38 Videos
- SOME BASIC CONCEPTS OF CHEMISTRYBook:R SHARMAChapter:SOME BASIC CONCEPTS OF CHEMISTRYExercise:Archives
Explore 26 Videos
Similar Questions
Knowledge Check
Consider the following reactions .
I . Zn(s)+CuSO4(aq)→
II . Fe(s)+ZnSO4(aq)→
III . Zn(s)+FeSO4(aq)→AI and IIBII and IIICI and IIIDAll of the aboveZn(s)+Cu2+(aq)→Zn2+(aq)+Cu(s) The cell representation for the above redox reaction is
ACu(s)∣∣Cu2+(aq)∣∣∣∣Zn2+(aq)∣∣Zn(s)BZn(s)∣∣Zn2+(aq)∣∣∣∣Cu2+(aq)∣∣Cu(s)CCu(s)∣∣Cu2+(aq)∣∣|Zn(s)|Zn2+(aq)DZn(s)∣∣Zn2+(aq)∣∣|Cu(s)|Cu2+(aq)What is the Gibbs energy of the following reaction?
Zn(s)+Cu2+(aq)→Zn2+(aq)+Cu2+(s),E∘cell=1.1VA106135.75Jmol−1B21227Jmol−1C−212.27kJmol−1D21227Jmol−1
Write Nernst equation for the following cell reaction :
Zn(s)∣∣Zn2+(aq)∣∣∣∣Cu2+(aq)∣∣Cu(s)
View SolutionComplete and balance the following chemical equations :
NaOH(aq)+Zn(s)→
View SolutionBalance the following half reactions in basis medium:
(a) CrO2−4(aq)→Cr(OH)Ө4(aq)
(b) CIOӨ(aq)→ClӨ(aq)
(c ) Bi3+(aq)→BiOӨ3(aq)
View SolutionA galvanic cell involves the following reaction:
Zn(s)+2Ag+(aq.)⇔Zn2+(aq.)+2Ag(s)
Select the correct statements among the following :
View SolutionConsider the following reactions.
।. Zn(s)+CuSO4(aq)→
II. Fe(s)+ZnSO4(aq)→
III. Zn(s)+FeSO4(aq)→
Which of the above chemical reaction(s) will take place?
View Solution
