How many moles of HI are needed to reduce one mole of carboxlic acid (RCOOH)into the corresponding alkane (RH)?
A
Two
B
Six
C
Four
D
Three
Video Solution
Text Solution
The correct Answer is:B
RCOOH+6HIRedP−−→ΔRCH3+2H2O+3I2
Note that each O atom is expelled as H2O. Thus, to remove two O atoms, we need 4H atoms (i.e., 4molofHI). Further, two H atoms are added to make it RCH3 ( two moles of HI). This makes a total of six moles of HI.
|
Similar Questions
Knowledge Check
How many moles of HI are needed to reduce one mole of aldehyde or keton?
AFourBTwoCSixDThreeHow many moles of HI are used to reduced one mole of monohydric alcohol ?
ASixBFourCThreeDTwoHow many moles of K2Cr2O7 are reduced by 1 mole of formic acid -
A1/3 molesB1 moleC2/3 moleD5/3 mole
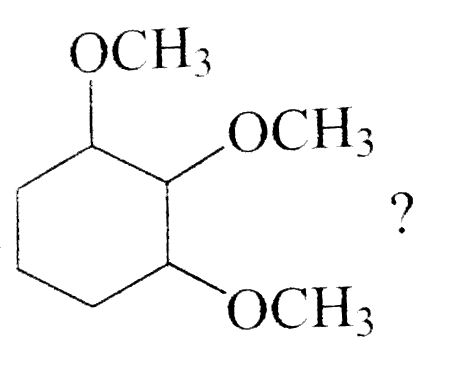
How many mole of 'HI' will react with
View SolutionHow many moles of nitrogen are needed to produce 8.2 moles of ammonia by reaction with hydrogen ?
View SolutionHow many moles of nitrogen are needed to produce 8.2 moles of ammonia by reaction with hydrogen ?
View SolutionHow many moles of FeC2O4 are required to reduce 2 " mol of "KMnO4 in acidic medium?
View SolutionHow many moles of KMnO4 are required to oxidise one mole of SnCl2 in acidic medium?
View Solution