Among the following groups of oxides, the group containing oxides that cannot be reduced by carbon to give the respective metals is
A
Cu2O,SnO2
B
Fe2O3,ZnO
C
CaO,K2O
D
PbO,Fe3O4
Text Solution
The correct Answer is:C
Ca and K are strong reducing agents, hence their oxides cannot be reduced with carbon.
|
Similar Questions
Knowledge Check
(viii) Amongest the following groups of oxides, the group containing oxides that cannot be reducing by carbon to give the respective metals is.
ACu2O,SnO2BFe2O3,ZnOCCaO,K2ODPbO,Fe3O4Oxides that cannot be reduced by C to give the respective metal are
ACaOandK2OBFe2O3andZnOCCu2OandSnO2DPbOandPb3O4Select the group of oxides that can not be reduced by carbon to give the respective matals.
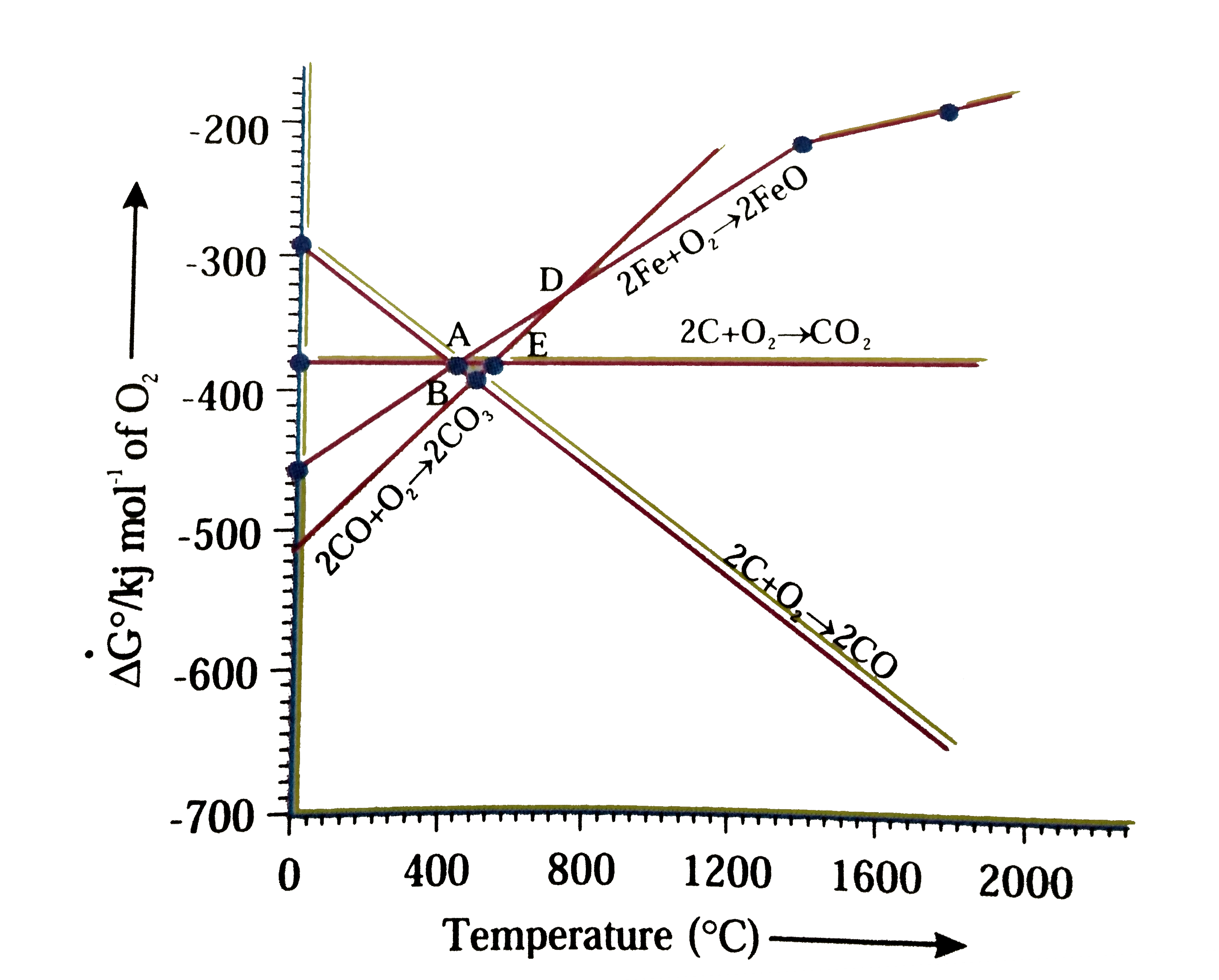 ACaO,K2OBFe2O3,ZnOCCu2O,SnO2DFe2O3,PbP.
ACaO,K2OBFe2O3,ZnOCCu2O,SnO2DFe2O3,PbP.
Which metal oxide cannot be reduced by carbon?
View SolutionWhich oxide cannot be reduced by H2?
View SolutionWhich of the following oxides cannot be reduced to metal by carbon
View SolutionWhich of the following oxide cannot be reduced to metal by carbon ?
View SolutionWhich of the following oxide cannot be reduced to metal by carbon ?
View Solution

