Select the group of oxides that can not be reduced by carbon to give the respective matals.
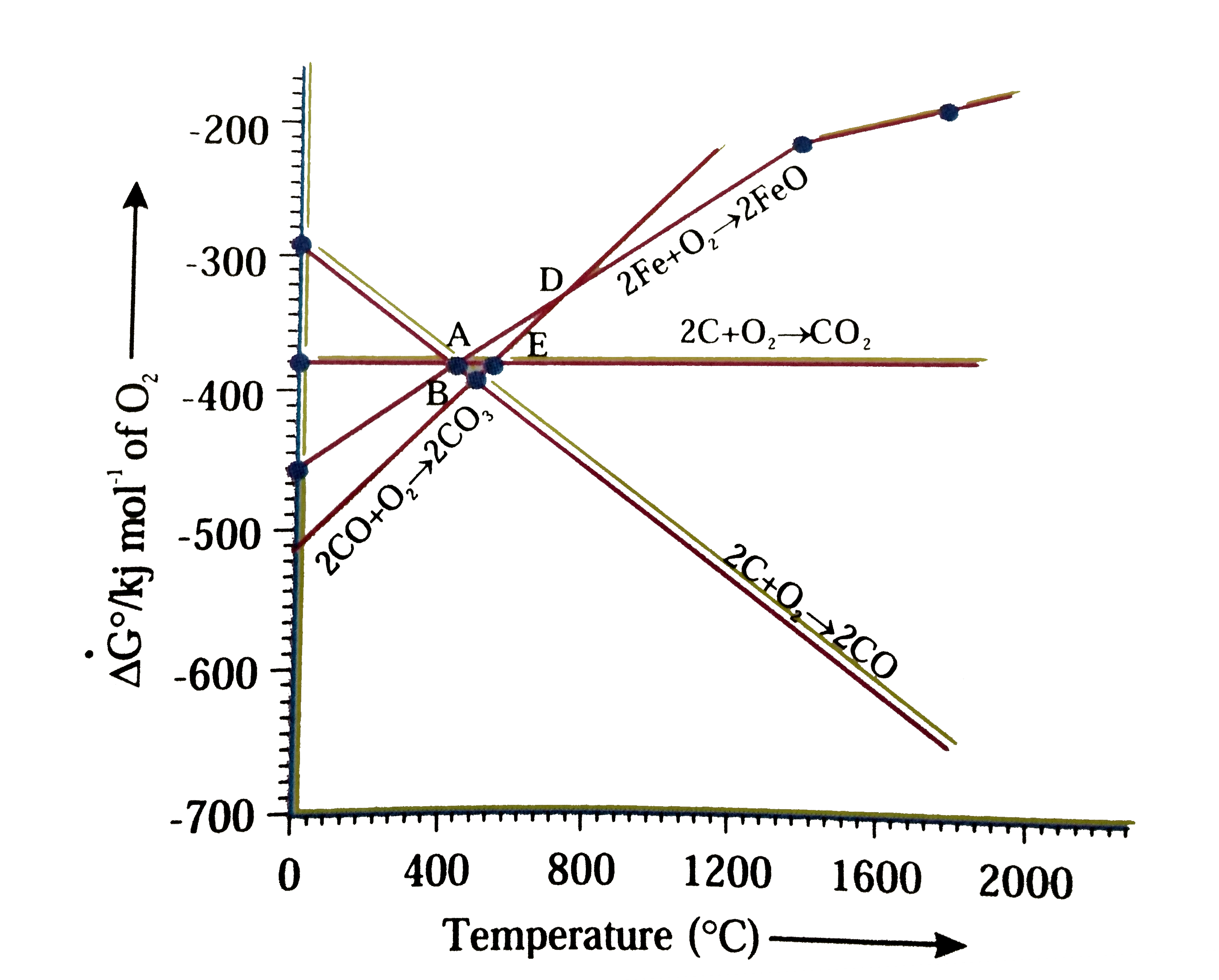
CaO,K2O
Fe2O3,ZnO
Cu2O,SnO2
Fe2O3,PbP.
The correct Answer is:A
Topper's Solved these Questions
- METTALURGYBOOK - NARAYNACHAPTER - METTALURGYEXERCISE - MULTIPLE ANSWER QUESTOINS
66 Videos
- METTALURGYBOOK - NARAYNACHAPTER - METTALURGYEXERCISE - Assertion and Reasoning Questions
2 Videos
- METTALURGYBOOK - NARAYNACHAPTER - METTALURGYEXERCISE - Concentration
6 Videos
- METALLURGYBOOK - NARAYNACHAPTER - METALLURGYEXERCISE - STATEMENT TYPE QUESTOINS
20 Videos
- NUCLEAR CHEMISTRYBOOK - NARAYNACHAPTER - NUCLEAR CHEMISTRYEXERCISE - INTEGER
28 Videos
Similar Questions
Froth flatiation
View SolutionStatement-1: The reduction of a metal oxide is easier if the metal formed is in liquid state at he temperature of reduction.
Statement-2: The value of entropy change ΔS of the reduction process is more on +ve side when the metal formed is in liquid state and the metal oxide being reduced in a solid state. Thus, the value of the ΔG becomes more or negative side.
View SolutionLeaching can be used for the extraction of which metals
(i) Pb (ii) Al (iii) Ag (iv) Au
View SolutionAssertion: All minerals are ore.
Reason: Ores are minerals from which metal can be extracted conveniently and economically.
View SolutionCalculate difference between number of a β particles emmited in the nuclear reaction
92U23→82Pb206
View Solution(viii) Amongest the following groups of oxides, the group containing oxides that cannot be reducing by carbon to give the respective metals is.
View SolutionSelect the group of oxides that can not be reduced by carbon to give the respective matals.

View SolutionAmong the following groups of oxides, the group that cannot be reduced by carbon to give the respective metals.
View SolutionAmong the following groups of oxides, the group containing oxides that cannot be reduced by carbon to give the respective metals is
View Solutionकुछ ऐसे धातुओं के ऑक्साइडों के उदाहरण दें जिनका अपचयन कार्बन के द्वारा किया जा सकता है।
View Solutionकार्बन कॉपर ऑक्साइड को अपचयित कर सकता है, परन्तु कैल्शियम ऑक्साइड को क्यों नहीं?
View Solution(viii) Amongest the following groups of oxides, the group containing oxides that cannot be reducing by carbon to give the respective metals is.
View Solutionऑक्साइडों के निम्न समूहों में ऑक्साइड युक्त वह कौनसा समूह है जिससे सम्बन्धित धातु को प्राप्त करने के लिये उसका कार्बन द्वारा अपचयन नहीं किया जा सकता
View SolutionThe group containing oxides that cannot be reduced by carbon to give the respective metal is :
View SolutionAmong the following group of oxides, the group containing oxides that cannot be reduced by carbon to give the respective metals , is :
View Solution

