Which of the following is the weakest acid?
A
p− Aminophenol
B
m− Aminophenol
C
o− Aminophenol
D
Phenol
Video Solution
Text Solution
The correct Answer is:A
in the ortho and para positions, NH2 is electron donating by resonance and is acid weakening. In the meta position it is only electron withdrawing and acid strengthening by induction. Thus, m-aminophenol is expected to be strongest acid but the decreasing order of acid strength is
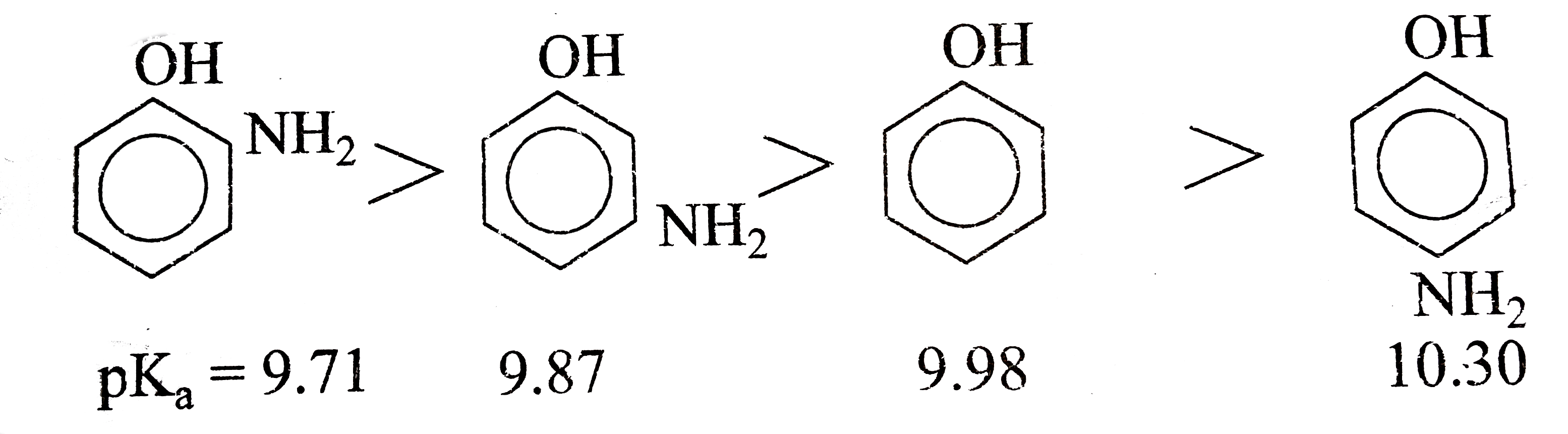
o- aminophenol is the strongest acid because its phenoxide ion is stabilized by intramolecular hydrogen bonding

p− Aminophenol is the weakest acid on acoount of least-Ieffect⋅.
|
Similar Questions
Knowledge Check
Which of the following is the weakest bond?
AHydrogen bondBCovalent bondCIonic bondDMetallic bondWhich of the following is the weakest base?
AMg(OH)2BCa(OH)2CNaOHDKOHWhich of the following is the weakest base?
ANaOHBCa(OH)2CNH4OHDKOH
