Which of the following is the strongest acid ?
A
Phenol
B
o−Methoxyphenol
C
m−Methoxyphenol
D
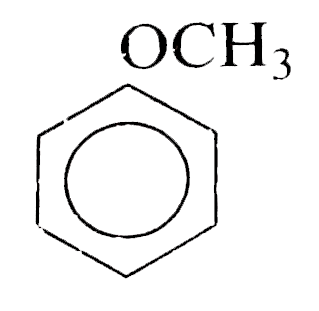
p−Methoxyphenol
Video Solution
Text Solution
The correct Answer is:C
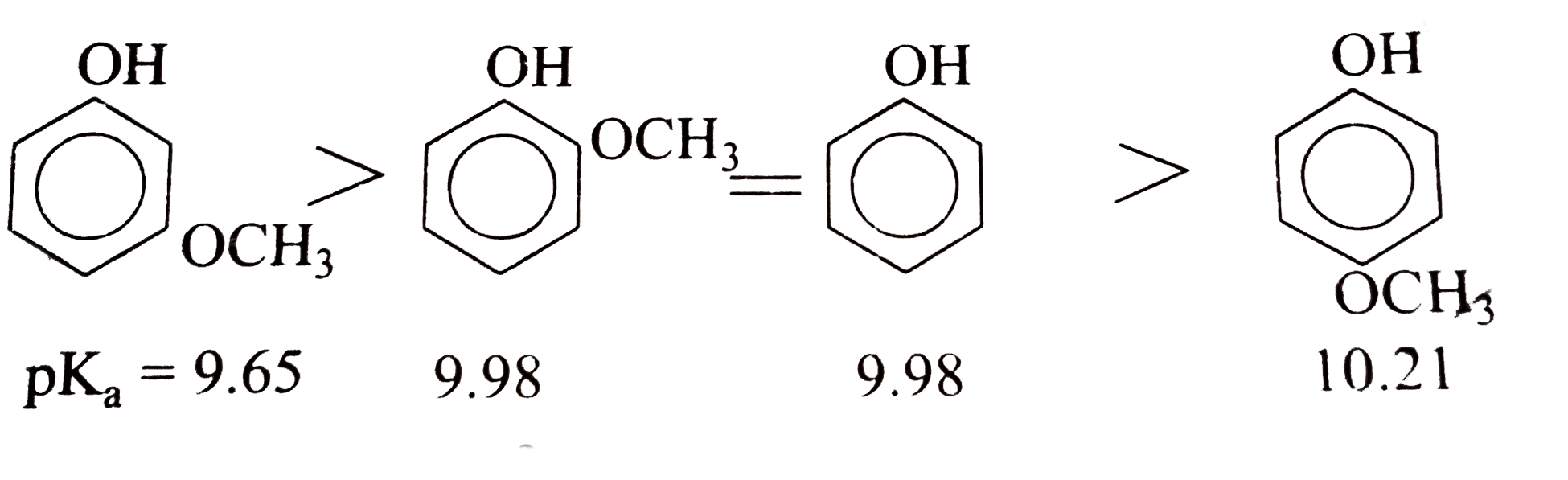
−Ieffect(o>m>p),+Reffect(o≈p,¬m),+Reffect>−Ieffect
Group like OCH3 with a+R effect weaken the phenol's acidic strength when in the o− position or p−position but strengthen its acidic strength when in the m−position. In the latter case, only the −Ieffect can opereate. Thus, the decreasing order of acidity is
Since the −Ieffectof−OCH3 group is more than that of NH2 group, m−methoxyphenol is a stronger acid than m−aminophenol.
|
Similar Questions
Knowledge Check
Which is the following is the strongest acid ?
A3,5-dinitrophenolB2,4-dinitrophenolCphenolD2,4,6-trinitrophenolWhich of the following is the strongest π−ac for π−aep→r ?
AF−C=C−FBH−C≡C−HCH2C=CH2DH−C≡C−NH2Which of the following is the strongest π−ac for π−acceptor ?
AF−C=C−FBH−C≡C−HCH2C=CH2DH−C≡C−NH2
Which one of the following is the strongest acid?
View SolutionWhich one of the following is the strongest acid ?
View SolutionWhich one of the following is the strongest acid ?
View SolutionWhich one of the following is the strongest acid -
View SolutionWhich one of the following is strongest acid ?
View Solution

