Dioxygen is not evolved when conc. H2SO4 reacts with
A
MnO3
B
CuSO4
C
KMnO4
D
K2Cr2O7
Video Solution
Text Solution
The correct Answer is:B
CuSO2+H2SO4→No reaction
2MnO2+2H2SO4→2MnSO4+2H2O+O2
4KMnO4+6H2SO4+6H2SO4Δ−−→2K2SO4+4MnSO4+6H2O+SO2
2K2Cr2O7+8H2SO4Δ−−→2K2SO4+2Cr2(SO4)3+8H2O+3O2
We can add H2O2 to acidified KMnO4 solution:
2KMnO4+3H2SO4→K2SO4+2MnSO4+3H2O+5[O]H2O2+O→H2O+O2×52KMnO4+3H2SO4+5H2O2→K2SO4+2MnSO4+8H2O+5O2
|
Similar Questions
Knowledge Check
Conc. H2SO4 is not
AHygroscopicBDehydrating agentCSulphonating agentDefflorescentAnisole with conc. H2SO4 gives.
APhenolBo-Phenol sulphonic acidCo-and p-sulphoanisaolesDm-sulphoanisoleWhich gas is evolved when H2O2 reacts with chlorine ?
AH2BO2CCIO2DNone of these
Kl when heated with conc .H2SO4 , it forms
View SolutionH2S gas is not evolved when SO2−3 ion reacts with following reagents.
View SolutionWhen
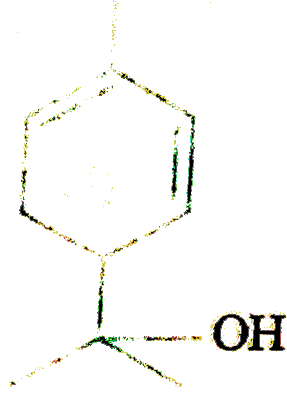 is reacted with conc. H2SO4 and heated then the intermediates and products formed are
is reacted with conc. H2SO4 and heated then the intermediates and products formed are
View SolutionAssertion : Dehydration of ethanol with conc. H2SO4 yields ethene.
Reason : The reaction of ethanol with conc. H2SO4 is known as dehydration.
View SolutionWhen formic acid is heated with concentrated H2SO4, the gas evolved is
View Solution

_S01_475_Q01.png)