H2S gas is not evolved when SO2−3 ion reacts with following reagents.
A
An+dil.H2SO4
B
Al + conc. NaOH
C
Al + dil. HCl
D
None of these
Text Solution
The correct Answer is:B
SO2−3+2Al+2OH−+3H2O→S2−+2[Al(OH)4]−
H2S does not evolve as liberated H2S is neutralised by NaOH and Na2S is formed.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which gas is evolved when H2O2 reacts with chlorine ?
AH2BO2CCIO2DNone of theseNitrogen gas is evolved when HNO2 reacts with .
AC6H5COCH2BC6H5CONH2CCH3CH2NH2DNH2CONH2Dioxygen is not evolved when conc. H2SO4 reacts with
AMnO3BCuSO4CKMnO4DK2Cr2O7
No. of moles of H2 gas evolved when one mole of the following compound reacts with sodium.
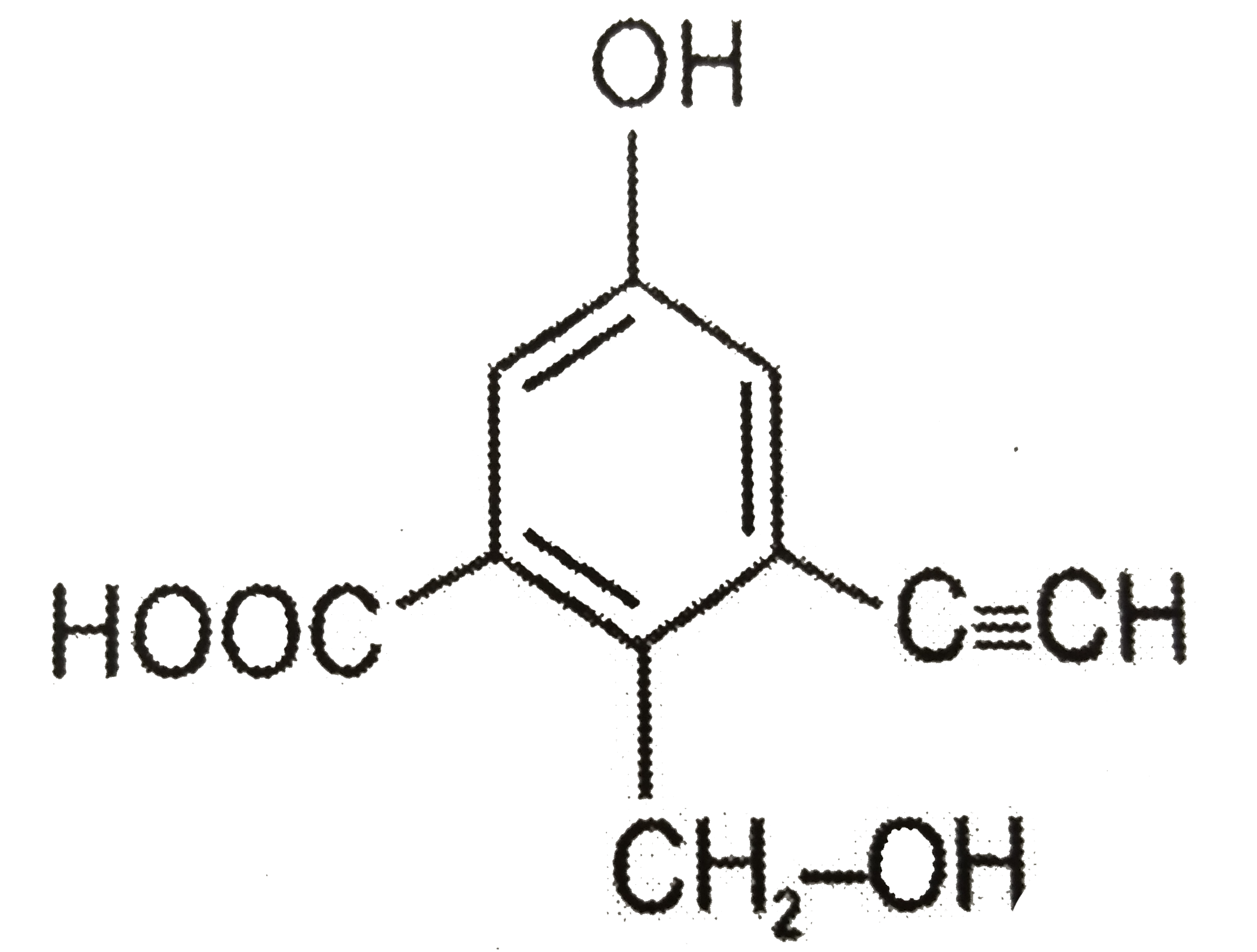
View SolutionNo. of moles of H2O gas evolved when one mole of the following compound reacts with sodium.
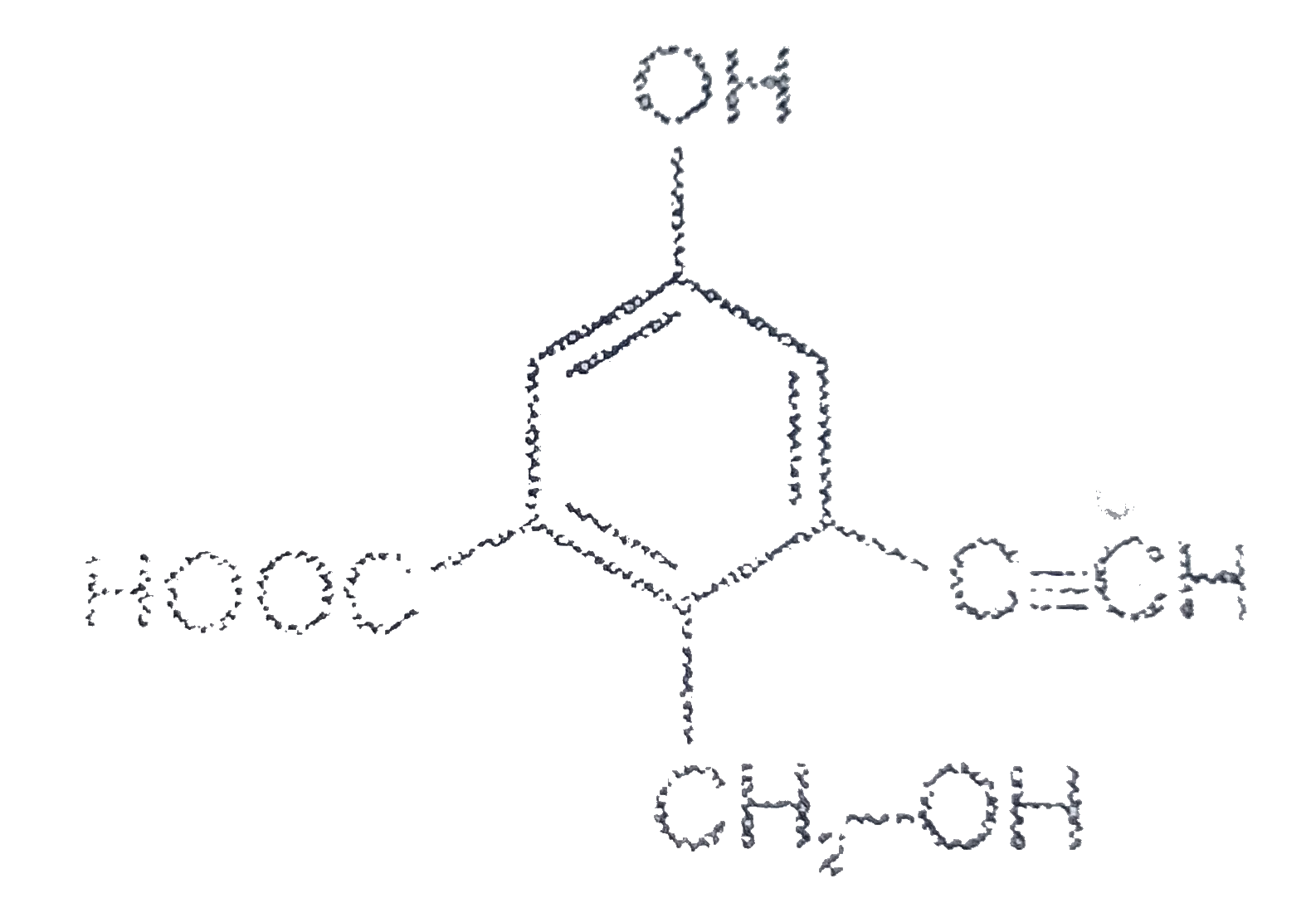
View SolutionNo. of moles of H2 gas evolved when one mole of the following compound reacts with sodium.

View SolutionWhat happens when H2S reacts with SO2 ?
View SolutionHydrogen gas is liberated when dil. H2SO4 react with
View Solution

