Consider the following atomic orbitals :
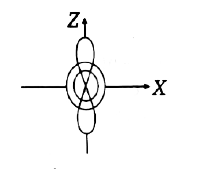
Which of the following statement(s) is /are correct regarding given orbital ?
It is a gerade atomic orbital
It has zero nodal plane
Circular electron density is present in XY plane
Opposite lobes of orbital have same sign of wave function (ψ)
The correct Answer is:A, B, C, D
It has two angular nodes modified in shape of nodal cones.
One lobe is dumb-bell shape while the other is in the shape of circular ring (in XY plane)

Topper's Solved these Questions
- CHEMICAL BONDING (BASIC)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - MATCH THE COLUMN
25 Videos
- CHEMICAL BONDING (BASIC)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - ASSERTION-REASON TYPE QUESTIONS
24 Videos
- CHEMICAL BONDING (BASIC)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - Level 3 (Passive 12)
4 Videos
- CHEMICAL BONDING (ADVANCED)BOOK - VK JAISWALCHAPTER - CHEMICAL BONDING (ADVANCED)EXERCISE - SUBJECTIVE PROBLEMS
72 Videos
- CO-ORDINATION COMPOUNDSBOOK - VK JAISWALCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - ASSERTION-REASON TYPE QUESTIONS
26 Videos
Similar Questions
Column-I and Column -II contains four entries each. Entries of Column-I are to be matched with some entries of Column-II. One or more than one entries of Column-I may have the matching with the same entries of Column-II.
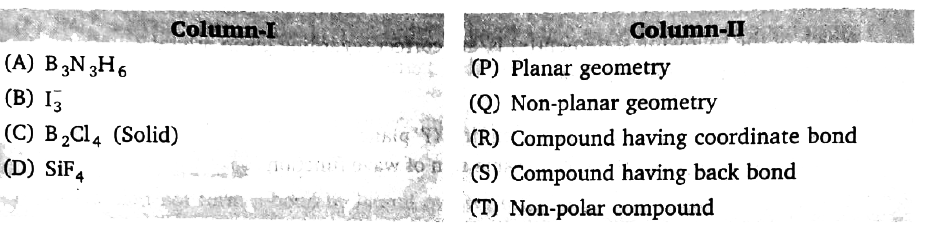
View SolutionAssertion : Multiple bond between two bonded atoms can have more than three bonds.
Reason : Multiple bond between two bonded atoms can not have more than two π-bonds.
View SolutionHybridisation involves the mixing of orbitals having comparable energhies of same atom. Hybridised orbitals perform efficient overlapping than overlapping by pure s, p or orbitals.
Which of the following is not correctly match between given species and type of overlapping ?
View SolutionThere are two groups of compounds A and B. Groups A contains three compounds Px4,Qy3,Rz2. Groups B also contains three compounds Sx4,Ty3,Uz2. Hybridization of each central atom of group A compounds is same as that of iodine in IBrCl− while in group B compounds it is same as that of iodine IBrCl+. Substituents X, Y and Z exhibit covalency of one in ground state. Then find the value of x/y.
Where, x and y are total number of lone pair present at central atoms of compounds of group A and B respectively.
View SolutionAssertion: In N2 Molecule, any N-atom can coordinate with central atom/ion.
Reason: N2 molecule can also act as ambidentate ligand.
View SolutionWhich orbit of the following statement (s) is//are correct ?
View SolutionThe correct statement regarding the third orbit of hydrgen atom is
View SolutionWhich of the following statements regarding an orbital are correct
View SolutionFew statements are given regarding nodes in the orbitals. Mark the statement which is not correct.
View SolutionWhich of the following is not a correct statement regarding the energies of orbitals?
View SolutionConsider the following three orbitals :
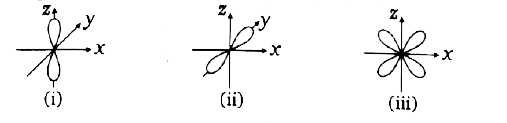
Correct statement(s) regarding given information is /are :
View SolutionWhich of the following statement regarding an orbital is correct ?
View SolutionConsider the following three orbitals : Correct statement(s) regarding given information is /are :
View SolutionConsider the following atomic orbitals :

Which of the following statement(s) is /are correct regarding given orbital ?
View SolutionWhich of the following statements regarding an orbital are correct
View Solution

