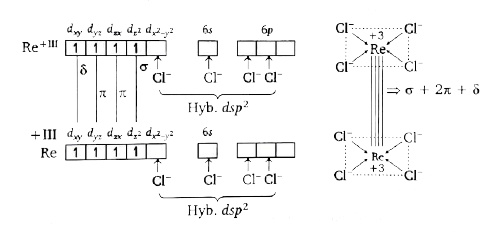
Reason: Complex anion [Re2Cl8]2− has one δ-bond, one sigma and one π-bond.
Reason:dz2 orbital can never form δ-bond.
A
If assertion is true but the reason is false
B
If assertion is false but reason is true
C
If both assertion and reason are true and the reason is the correct explanation of assertion
D
If both assertion and reason are true but reason is not the correct explanation of assertion
Video Solution
Text Solution
The correct Answer is:B
|
Similar Questions
Knowledge Check
Number of sigma and pi bonds in ClO−2 ion
A4σand4πB4σand2πC4σand3πD3σand2πNumber of sigma and pi bonds in ClO−2 ion
A2σand2πB2σand1πC1σand2πD3σand2πNumber of sigma and pi bonds in ClO−2 ion
A2σand2σB3σand3πC3σand2πD2σand3π
Why are pi-bonds formed in a molecule ? Can d-orbitals also form pi-bonds ?
View SolutionOut of σ and π− bonds, which one is stronger and why?
View SolutionIn which at least one σ bond of np(σnp) orbital is present :-
View SolutionThe ratio of number of σ -bond to π− bond in N2 and CO2 molecules are
View SolutionWhich of the following orbital can not form π as well as δ-Bond ?
View Solution