All alkali metals dissolve in anhydrous liquid ammonia to give blue colour solution. It is the ammoniated electron which is reponsible for the blue colour of the solution, and the electrical conductivity is due to the ammoniated cation, [M(NH3)x]+ as well as the ammoniated electron,[e(NH3)y]−, value of x and y depend on the extent of solvation by NH3. Dilute solutions are paramagnetic due to free ammoniated electrons.
Q. Ammoniated solutions of alkali metals are reducing agents due to the presence of free ammoniated or solvated electrons that can reduce:
(I)O2toO2−2 (II) K2[Ni(CN)4] to K4[Ni(CN)4]
(III) Aromatic ring (IV) Non-terminal alkyne
Choose the correct code:
III and IV
II and III
I, II, III and IV
I, III and IV
The correct Answer is:C
M(s)+NH2excess(l)→M+(NH3_(2)+¯e(NH3)2
ammoniated ¯e responsible for blue colour and reducing character.
If conc. Of solution is increased, then associated of solvated electrons gas started hence, paramagnetism decrease and solution changes to bronze colour.
Topper's Solved these Questions
- s-BLOCK ELEMENTSBook:VK JAISWALChapter:s-BLOCK ELEMENTSExercise:ONE OR MORE ANSWER IS/ARE CORRECT
Explore 69 Videos
- s-BLOCK ELEMENTSBook:VK JAISWALChapter:s-BLOCK ELEMENTSExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 34 Videos
- s-BLOCK ELEMENTSBook:VK JAISWALChapter:s-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 2 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWALChapter:QUALITATIVE INORGANIC ANALYSISExercise:SUBJECTIVE PROBLEMS
Explore 5 Videos
- TYPES OF REACTIONSBook:VK JAISWALChapter:TYPES OF REACTIONSExercise:SUBJECTIVE PROBLEMS
Explore 42 Videos
Similar Questions
The correct statement(s) is /are
View SolutionStatement-I : Li2SO4 do not form double salt like alum.
Statement-II : Li reacts with NH3 gas to form LiNH2
View Solution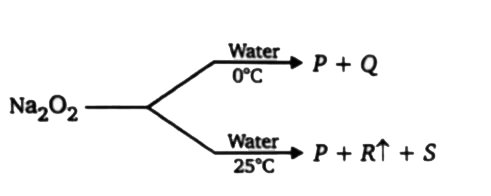
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionConsider the following reaction
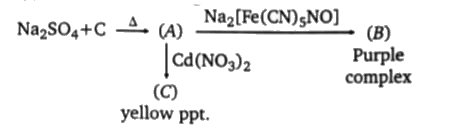
Then calculate value of ∣∣X2−Y2∣∣ (where X and Y are total number of electrons present t_(2g) and e_(g) orbitals respectively in d-block metal ion of compound B).
View SolutionFind total number of reagnets which cann produce I2 from KI solution.
Conc. H2SO4, Hg(NO3)2solution, CuSO4 solution, Conc.H3PO4,
K2Cr2O7/H+, Cl2 Water, Pb(CH3COO)2 solution, Ca(OCl)Cl/H+, NaNO2+dil.HCl
View SolutionDilute solutions of alkali metals in liquid NH_(3) are blue. It is the ammoniated electron which is responsible for the blue colour of the solution, and the electrical conductivity is due to the ammoniated cation, [M(NH_(3))_(x)]^(+) as well as the ammoniated electrons, [e(NH_(3))_(y)]^(-) , values of x and y depend on the extent of solvation (by NH_(3) ). Dilute solutions are paramagnetic due to free electrons. What happens if alkali metals is allowed to react with concentrated liquid ammonia?
View SolutionDilute solutions of alkali metals in liquid NH3 are blue. It is the ammoniated electron which is responsible for the blue colour of the solution, and the electrical conductivity is due to the ammoniated cation, [M(NH3)x]+ as well as the ammoniated electrons, [e(NH3)y]−, values of x and y depend on the extent of solvation (by NH3). Dilute solutions are paramagnetic due to free electrons.
Ammoniated solution of alkali metals are reducing agents due to the presence of free ammoniated or solvated electrons that can reduce:
[I]O2 to O2−2 [II]K2[Ni(CN)4] to K4[Ni(CN)4]
[III] aromatic ring [IV] non-terminal alkyne
Choose the correct code
View SolutionAssertion Alkaline earth metals dissolve in liquid ammonia to give a blue-black coloured solution. The blue-black colour of the solution is due to the presence of ammoniated electrons.
View SolutionSodium gives blue colour in NH3 solution. This blue colour is due to
View Solutionकथन : क्षार धातुओं के द्रव अमोनिया में बने विलयन उत्तम चालक होते हैं ।
कारण : क्षार धातुओं के द्रव अमोनिया में बने विलयनों में अमोनीकृत धनायन तथा अमोनीकृत इलेक्ट्रॉन उपस्थित होते हैं ।
View SolutionThe alkali metals dissolve in ammonia to give a deep blue solution which is cnducting in nature. M+(x+y)NH_(3)to[M(NH_(3))_(x)]^(2+)+2[e(NH_(3))_(y)]^(-) which of the followin is not true about the solutions of alkali metals in liquid ammonia?
View SolutionAll alkali metals dissolve in anhydrous liquid ammonia to give blue colour solution. It is the ammoniated electron which is reponsible for the blue colour of the solution, and the electrical conductivity is due to the ammoniated cation, [M(NH3)x]+ as well as the ammoniated electron,[e(NH3)y]−, value of x and y depend on the extent of solvation by NH3. Dilute solutions are paramagnetic due to free ammoniated electrons.
Q. Which of the following statement about solution of alkali metals in liquid ammonia is correct?
View SolutionAll alkali metals dissolve in anhydrous liquid ammonia to give blue colour solution. It is the ammoniated electron which is reponsible for the blue colour of the solution, and the electrical conductivity is due to the ammoniated cation, [M(NH3)x]+ as well as the ammoniated electron,[e(NH3)y]−, value of x and y depend on the extent of solvation by NH3. Dilute solutions are paramagnetic due to free ammoniated electrons.
Q. Which of the following statement about solution of alkali metals in liquid ammonia is correct?
View SolutionHow alkaline earth metal dissolve in liquid ammonia ?
View SolutionA : On reaction with liquid ammonia, alkali metals give conducing solution.
R : Ammoniated e− and ammoniated cations are formed when alkali metals is present in liquid ammonia.
View Solution

