The following data were reported for the decompoistion of N2O5 in CCl4 at 303K:
∣∣∣Time (min)120160200240∞Vol ofO2(mL)37.7045.8552.6758.3484.35∣∣∣
Show that the reaction is the first order and calculate the rate constant.
Note: This is an example of direct-estimation of Product, i.e., O2.
The correct Answer is:0.0049 minute−1
Step by step video, text & image solution for The following data were reported for the decompoistion of N_(2)O_(5) in C Cl_(4) at 303 K: |{:("Time (min)",120,160,200,240,oo),("Vol of" O_(2) (mL),37.70,45.85,52.67,58.34,84.35):}| Show that the reaction is the first order and calculate the rate constant. Note: This is an example of direct-estimation of Product, i.e., O_(2). by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Similar Questions
The following data were reported for the decompoistion of N2O5 in CCl4 at 303K:
∣∣∣Time (min)120160200240∞Vol ofO2(mL)37.7045.8552.6758.3484.35∣∣∣
Show that the reaction is the first order and calculate the rate constant.
Note: This is an example of direct-estimation of Product, i.e., O2.
View SolutionThe following data were obtained during the catalysed decomposition of N2O at 1173 K :
Time (min)30536380100120%age decomposed325057657378
Show that the reaction is of the first order and calculate velocity constant of the reaction.
View SolutionIn an experiment on the decompoistion of an aqueous NH4NO2 at a certain temperature, the volume of nitrogen gas collected at different intervals of time was as follows:
∣∣∣Time (s)11902300∞Vol ofN2(mL)5.007.309.50∣∣∣
Show how the above data illustrate that the reaction is of first order.
Note: This is an example of direct-estimation of Product, i.e., N2.
View SolutionThe following rate data were obtained for the thermal decomposition of N2O5(g)
2N2O5(g)→2N2O4(g)+O2(g)
Time (sec)050Total pressure (atm)0.20.25
Calculate the reaction rate when the total pressure is 0.28 atm
View Solutionform the following data for the decompoistion of N2O5 in carbon tertrachloride solution at 321K, show that the reaction is of the first order and calculate the rate constant.
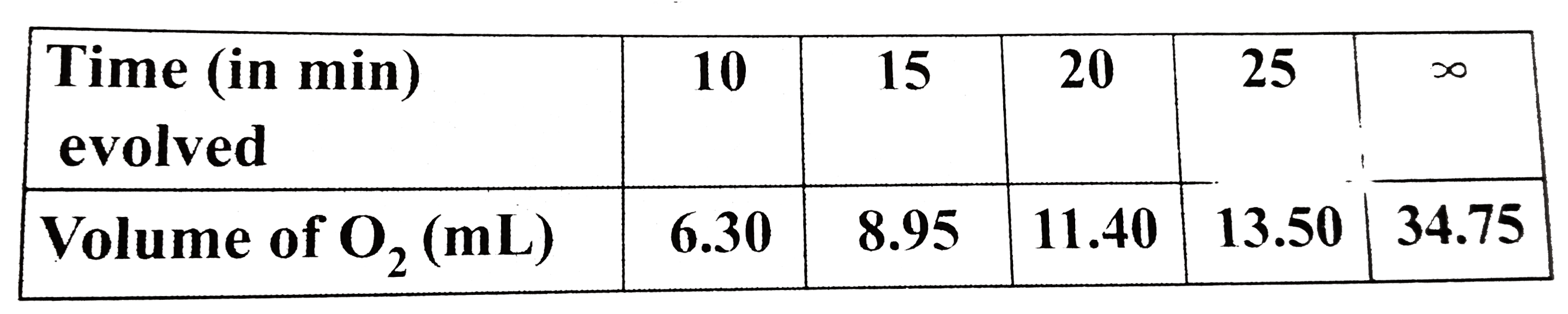
View SolutionFrom the following data for the decomposition of diazobenzene chloride , show that the reaction is of first order :
Time205070∞(min)Vol ofN2102533162(mL) .
View SolutionFollowing data were obtained during the thermal decompoistion of sulphuryl chloride at constant volume and 285∘C.
SO2Cl2(g)→SO2+Cl2(g)
∣∣∣Time (s)0240600Pressure (torr)180244302∣∣∣
Show that it is first order reaction.
View SolutionThe following data were obtained during the first thermal decompoistion of N2O5(g) at constant volume.
2N2O5(g)→2N2O4(g)+O2(g)
∣∣ ∣ ∣∣S.No.Time (s)Total pressure (atm)i.00.5ii.1000.512∣∣ ∣ ∣∣
Calculate the rate constant.
View SolutionFollowing data are obtained for the reaction:
N2O5→2NO2+.1/2O2
∣∣∣t/s0300600[N2O5]/molL−11.6×10−20.8×10−20.4×10−2∣∣∣
(a) Show that it follows first order reaction
(b) Calculate the half-life
(Given log 2 = 0.3010 log 4 = 0.6021]
View SolutionThe rate of decomposition of N2O5 in CCl4 solution has been studied at 318 K and the following results have been obtained :
t/min01353426831693c/M2.081.911.671.350.57
Find the order of the reaction and calculate its rate constant. What is the half-life period ?
View Solution
