Out of two bromoderivatives
C6H5CH(CH3)BrandC6H5CH(C6H5)Br, which one is more reactive in SN1 reaction and why?
In SN1 reaction, reactivity depends upon the stability of the intermediate carbocation. Thus,
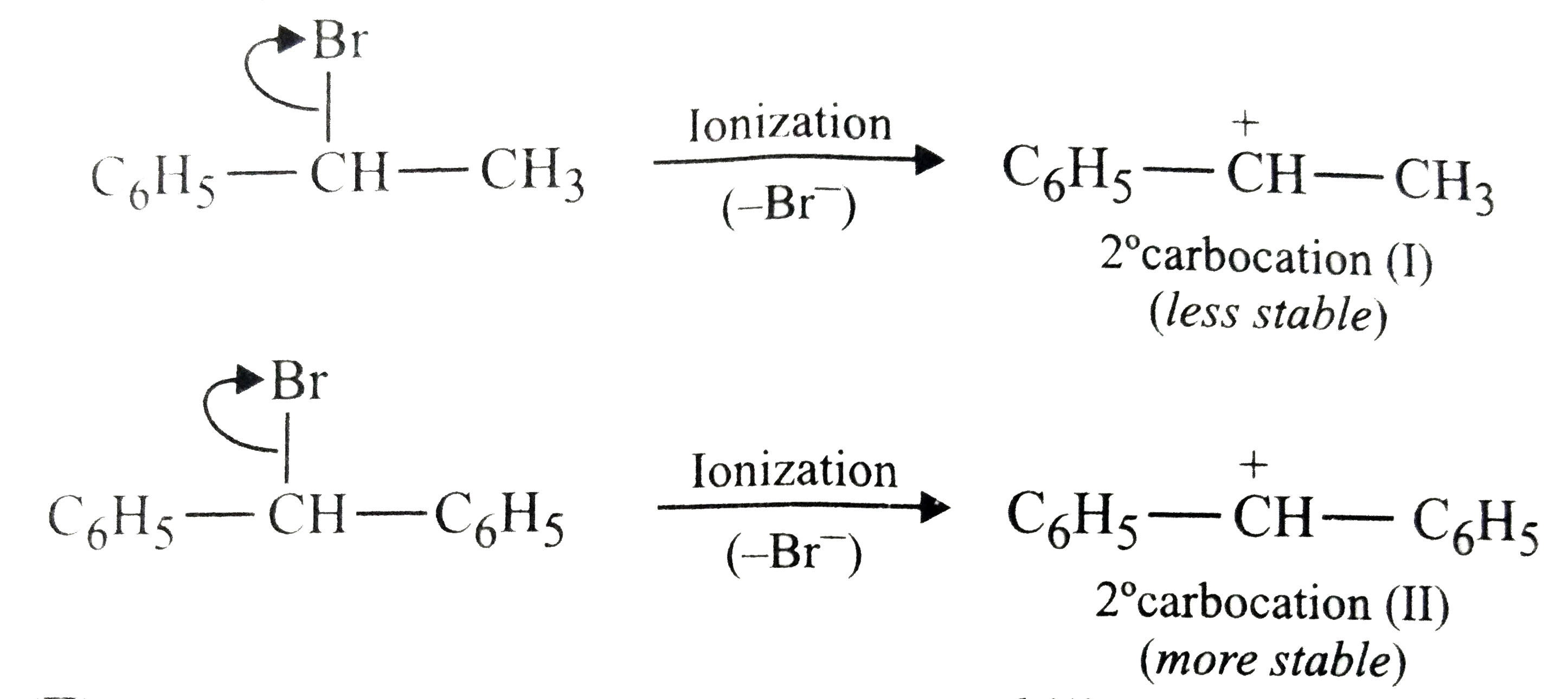
The carbocation (II) derived from C6H5CH(C6H5)Br is stabilized by +R-effect of the two C6H5 groups the carbocation (I) derived from C6H5CH(CH3)Br is stabilized by +R-effect of one C6H5 group and +I-effect of the CH3 group. since +R-effect is much stronger than +I-effect, therefore, carbocation (II) is more stable than carbocation (I). thus, C6H5CH(C6H5)Br is more reactive than C6H5CH(C6H5)Br is more reactive than C6H5CH(CH3)Br in SN1 substitution reaction.
Similar Questions
The decreasing order of reactivity of the following compounds in SN1 reactions is _________.
(i) C6H5CH2Br (ii)C6H5CH(C6H5Br
(iii)C6H5CH(CH3Br (iv)C6H5C(CH3)(C6H5)Br
View SolutionPredict the order of reactivity of the following compounds in S_(N)1 and S_(N)2 reactions: (i) the four isomeric bromobutanes (ii) C_(6)H_(5)CH_(2)Br,C_(6)H_(5)CH(C_(6)H_(5))Br,C_(6)H_(5)CH(CH_(3))Br,C_(6)H_(5)C(CH_(3))(C_(6)H_(5))Br .
View Solutionनिम्न के बीच पहचान कीजिये-
C6H5−CN=CH−COCH3 और C6H5−CH=CH−COCH2CH3 में
View SolutionPredict the order of reacitivity of the following compounds in S_(N)1 and S_(N)2 reactions: (2) C_(6)H_(5)CH_(2)Br C_(6)H_(5)(CH_3)Br, C_(6)H_(5)C(CH_3)(C_(6)H_5)Br .
View SolutionThe number of reactions proceeding through free radical mechanism are
(i) C2H5Br+Na+−OCH3
(ii) C2H5Br+AgCN
(iii) C6H5CH3+Cl2(hv)
(iv) CH3CH=CH2+HBr (peroxides)
(v) C2H5Br+alc.KOH
(vi) CH3CH=CH2+HCl(peroxide)
(vii) C2H5Br+H2(NiorPd)
(viii) C2H6+Cl2(hv)
(ix) C2H5Br+NaSH
View SolutionPredict the order of reactivity of the following compounds in SN1 and SN2 reactions :
(a) The four isomeric bromobutanes
(b) C6H5CH2Br,C6H5CH(C6H5)Br,C6H5CH(CH3)Br,C6H5C(CH3)(C6H5)Br
View SolutionThe number of reactions proceeding through free radical mechanism are
(i) C2H5Br+Na+−OCH3
(ii) C2H5Br+AgCN
(iii) C6H5CH3+Cl2(hv)
(iv) CH3CH=CH2+HBr (peroxides)
(v) C2H5Br+alc.KOH
(vi) CH3CH=CH2+HCl(peroxide)
(vii) C2H5Br+H2(NiorPd)
(viii) C2H6+Cl2(hv)
(ix) C2H5Br+NaSH
View Solution

