Which of the following compounds have approximately the same dipole moment?
Text Solution
The correct Answer is:A, B, D
In option (d), the dipole moment of the two para Cl atoms cancel each other, therefore, its dipole moment is the same as that of 1,3-dichlorobenzene. But the dipole moment of 1,3-dichlorobenzene is the same as that of chlorobenzene due to parallelogram law of forces.
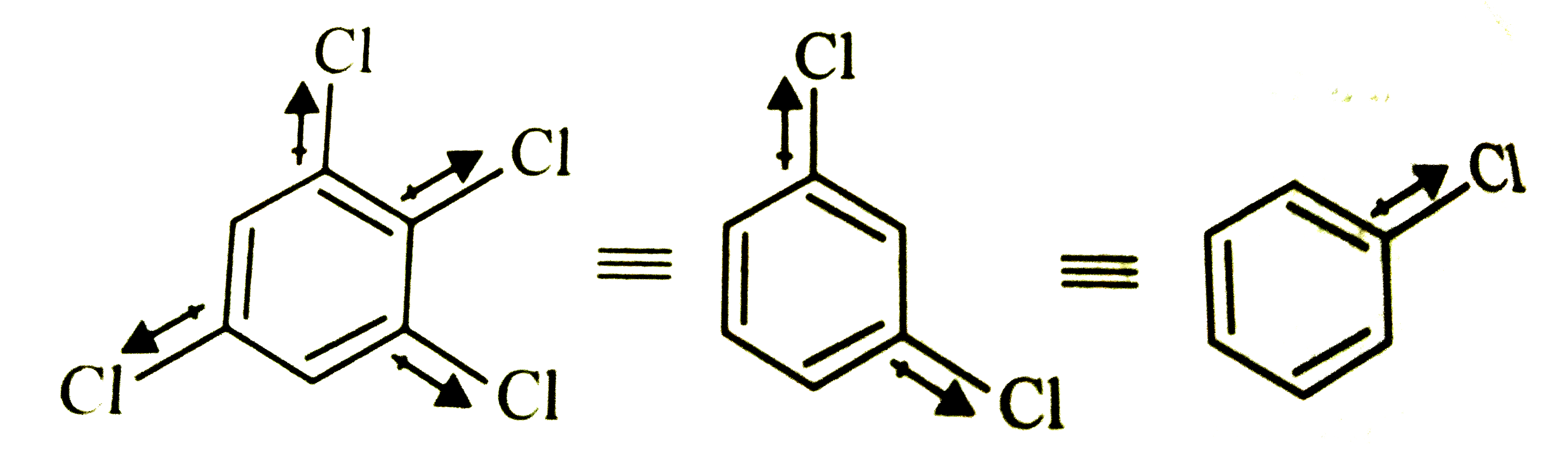 .
.
|
Similar Questions
Knowledge Check
Which of the following compounds have a dipole moment?
A B
B C
C D
D
Which of the following compounds have zero dipole moment ?
ABF3BSnCl2CH2ODNH3Which of the following compounds have finite dipole moments ?
Atrans-2-PenteneBcis-2-PenteneC1-ButyneD2-Butyne
Which of the following compounds would you expect to have a dipole moment ?
View SolutionWhich of the following compounds will have the highest dipole moment ?
View SolutionWhich one of the following is a compound most likely to have a dipole moment
View SolutionWhich of the following compounds would you expect to have a dipole moment ?
View SolutionWhich of the following compounds has dipole moment approximately equal to that of chlorobenzene?
View Solution

