Arrange the following compounds in decreasing order of acidity.
H2O,ROH,HC≡CH
Video Solution
Text Solution
A stronger acid displaces a weaker acid from its salt. When RONa is treated with H2O, it forms ROH, so water is a stronger acid than ROH.
HOHStronger acid+RONa→NaOH+ROHWeaker acid
Similary, when sodium ethynide is treated with water and alcohol, then acetylene is obtained.
HOHStronger acid+CH≡CNa→HC≡CHWeak acid+NaOH
ROHStronger acid+HC≡CNa→HC≡CHWeak acid+RONa
Thus, water and aclohol are strongest acid than ethyne and the decreasing order of acidity of given compounds are
HOH>ROH>HC≡CH
|
Similar Questions
Knowledge Check
Arrange the following compounds in decreasing order of acidity.
H2O,ROH,HC≡CHAH2O>HC≡CH>ROHBH2O>ROH>HC≡CHCHC≡CH>ROH>H2ODHC≡CH>H2O.ROHArrange the following compounds in decreasing order of basicity :
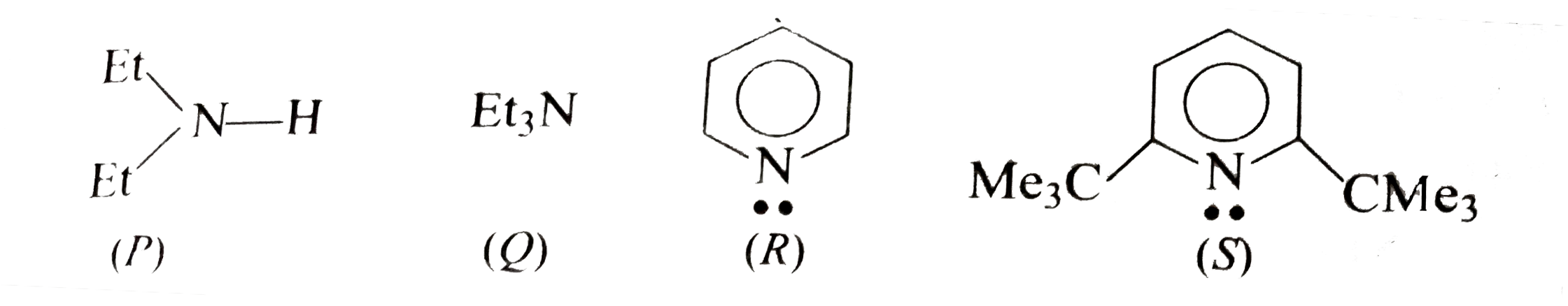 AP>Q>R>SBQ>P>R>SCR>S>Q>PDQ>P>S>R
AP>Q>R>SBQ>P>R>SCR>S>Q>PDQ>P>S>RArrange the following compounds in decreasing order of acidity :
CH3CH2OH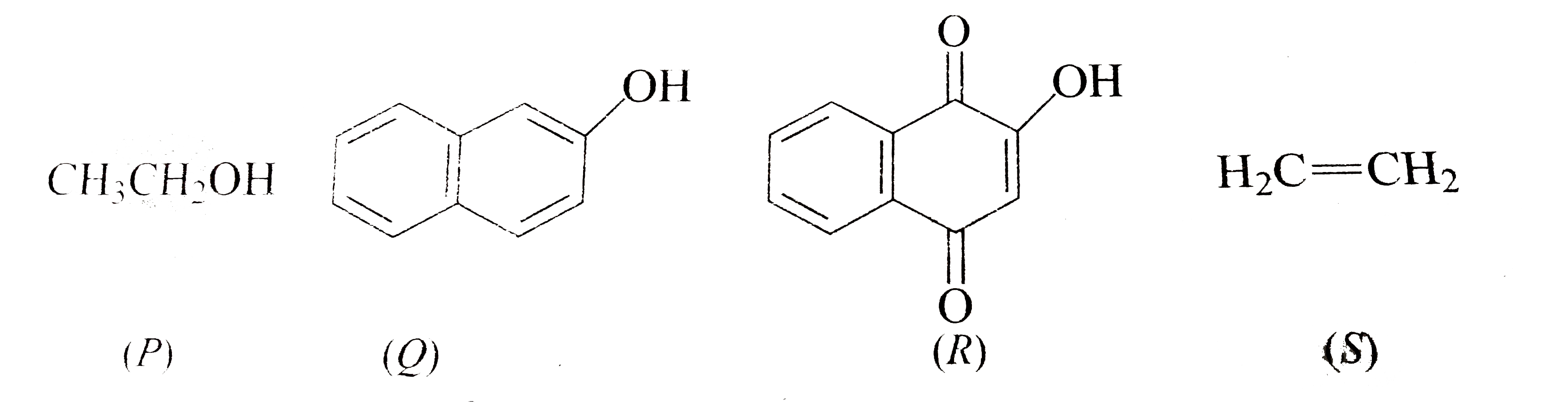
H2C=CH2AR>Q>P>SBQ>R>P>SCS>R>Q>PDQ>P>R>S
Arrange the following compounds in order of decreasing acidity:
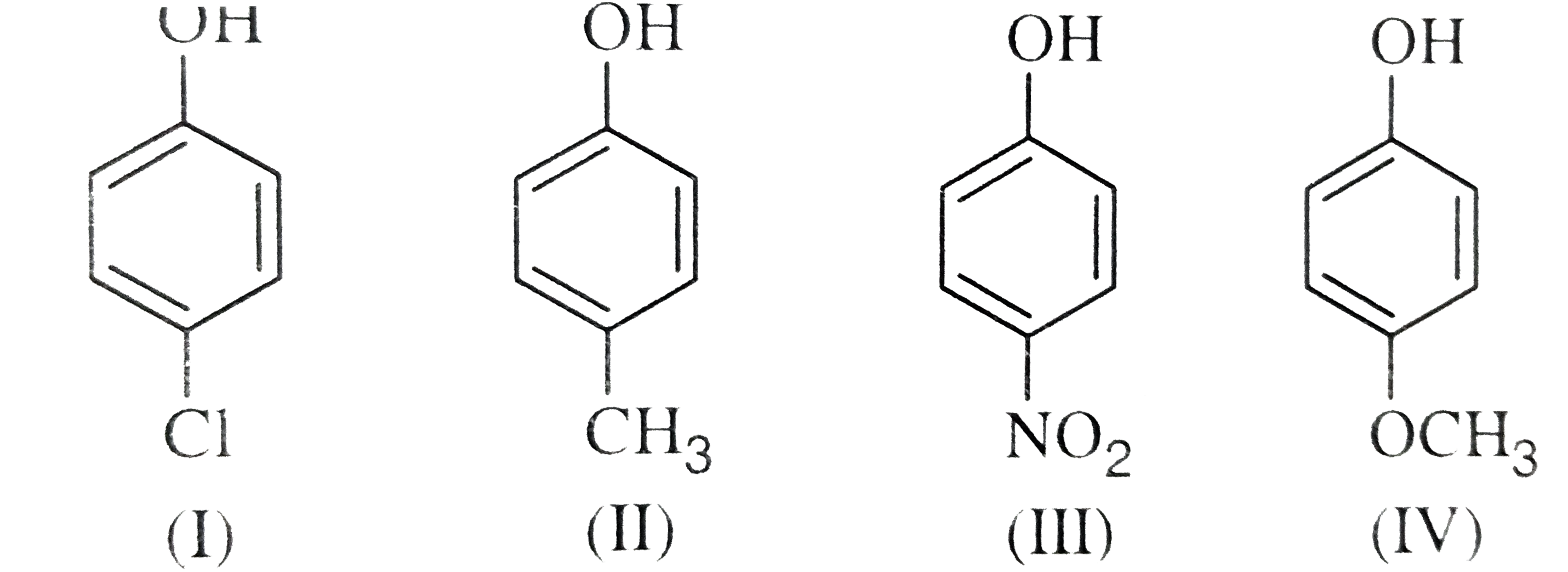
View SolutionArrange the following compound in decreasing order of boiling point
View SolutionArrange the following compounds in order of decreasing acidity:
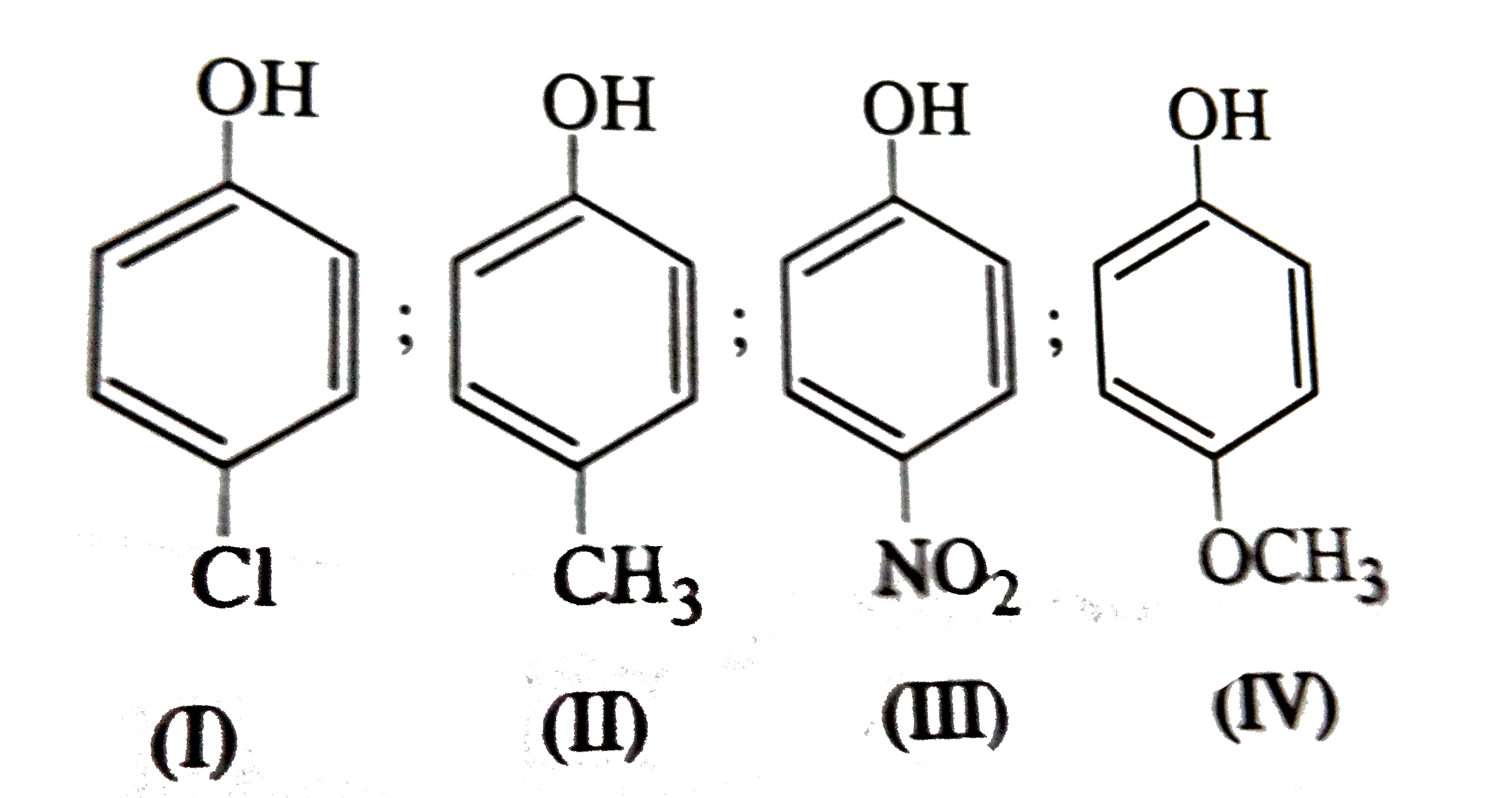
View SolutionArrange the following compounds in order of decreasing acidity :
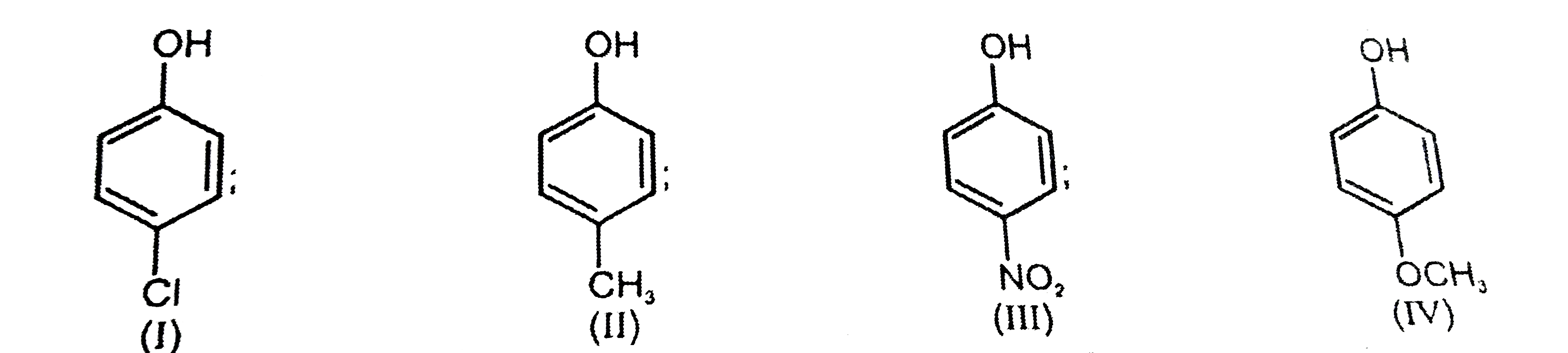
View SolutionArrange the following compounds in order of decreasing acidity :
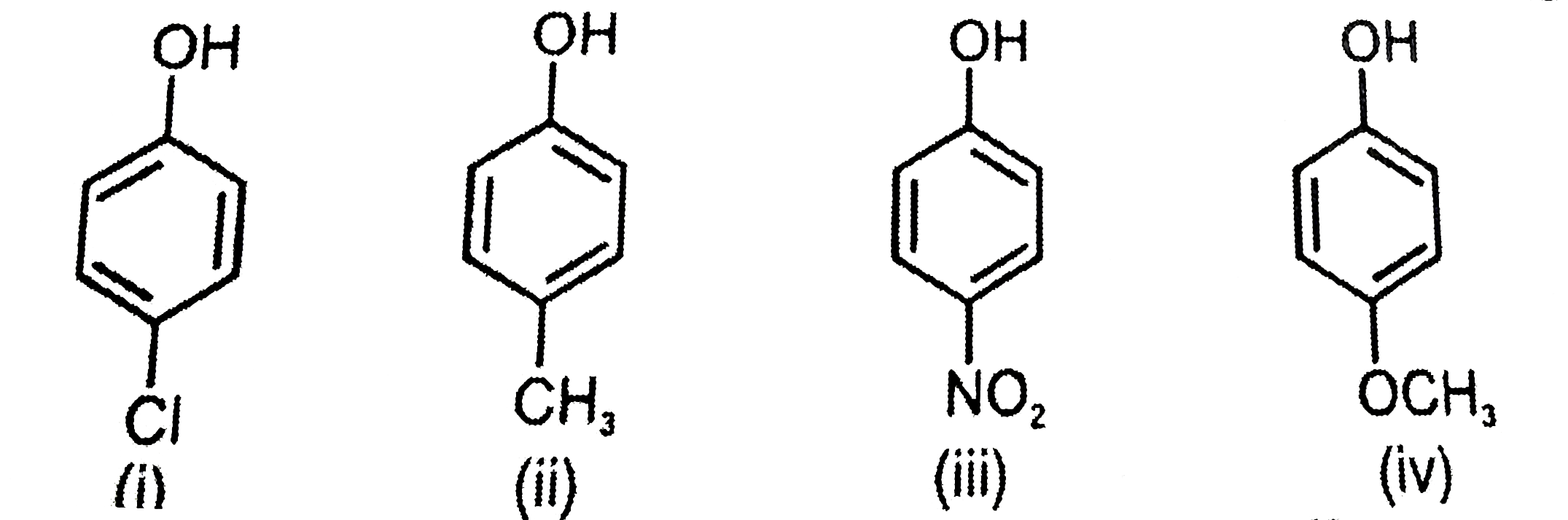
View Solution

 .
.