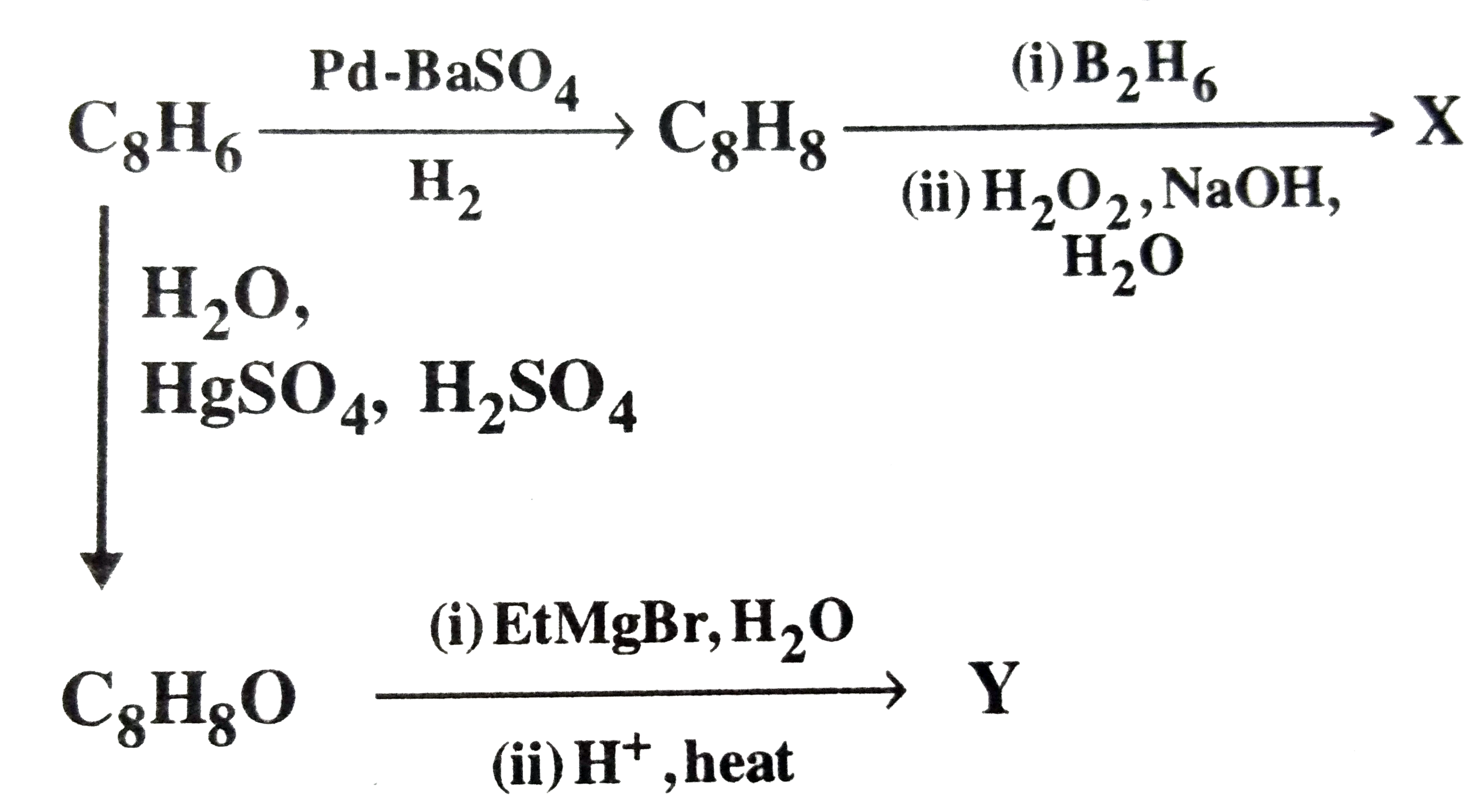
Q. Compound X is
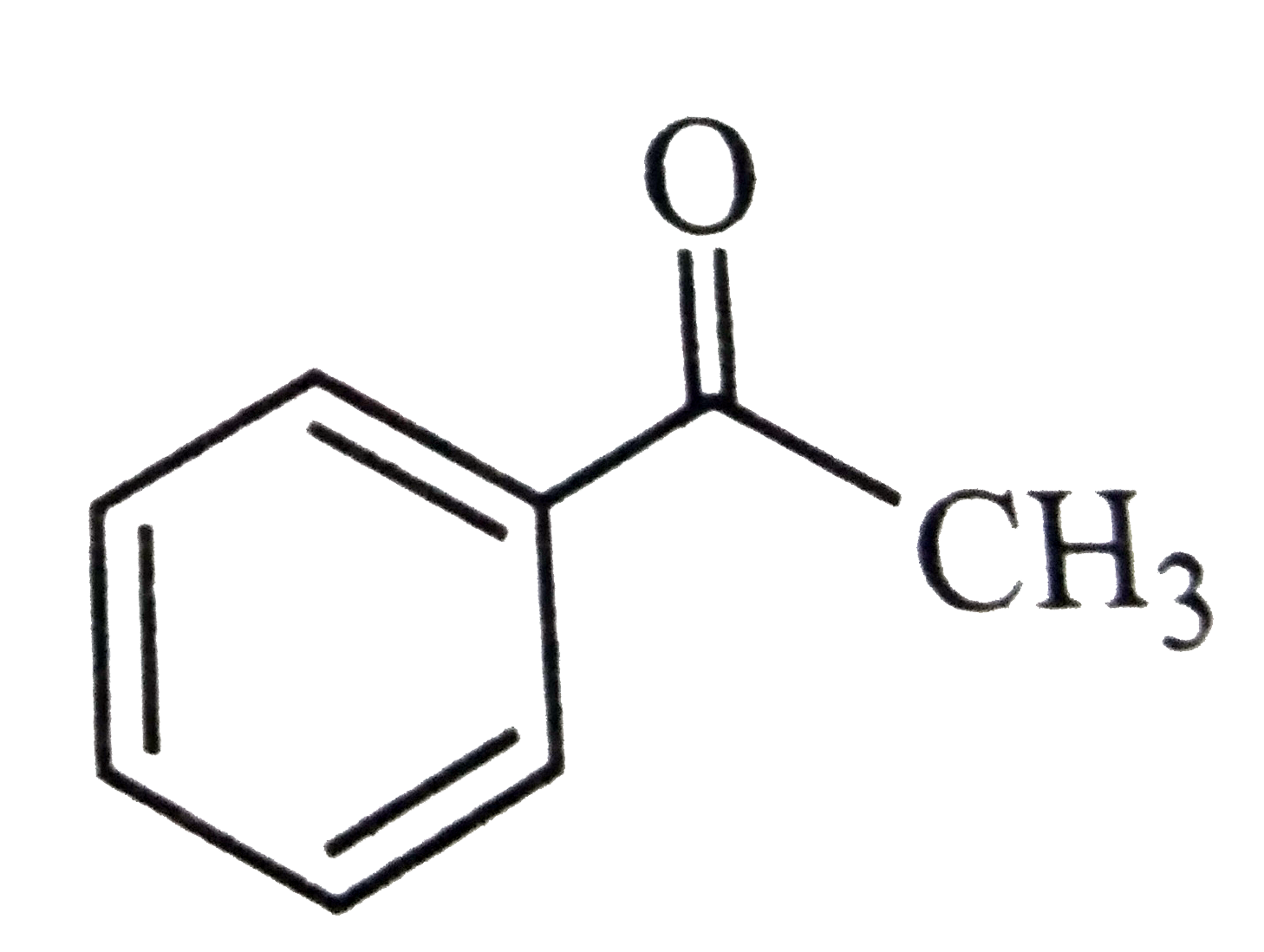
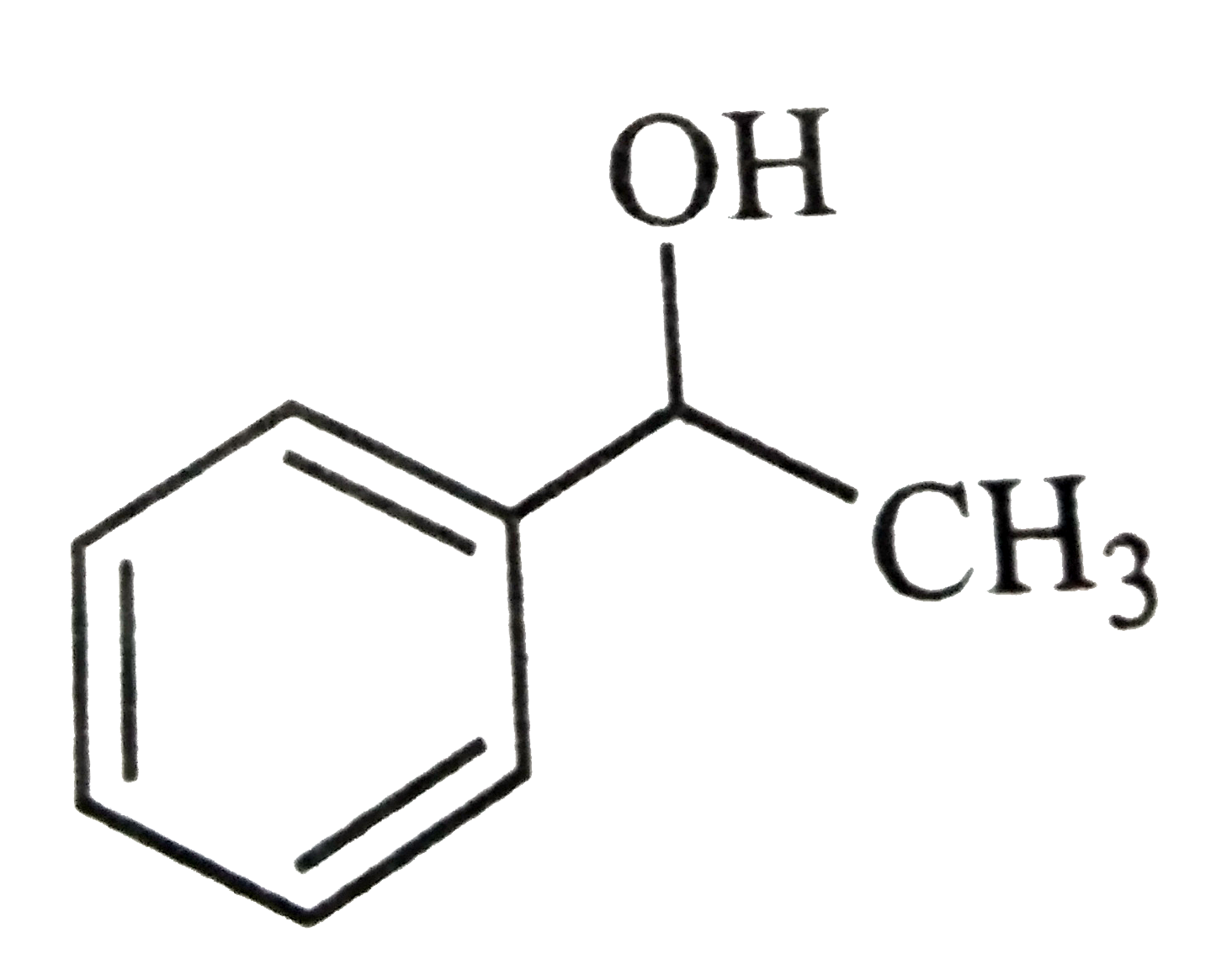
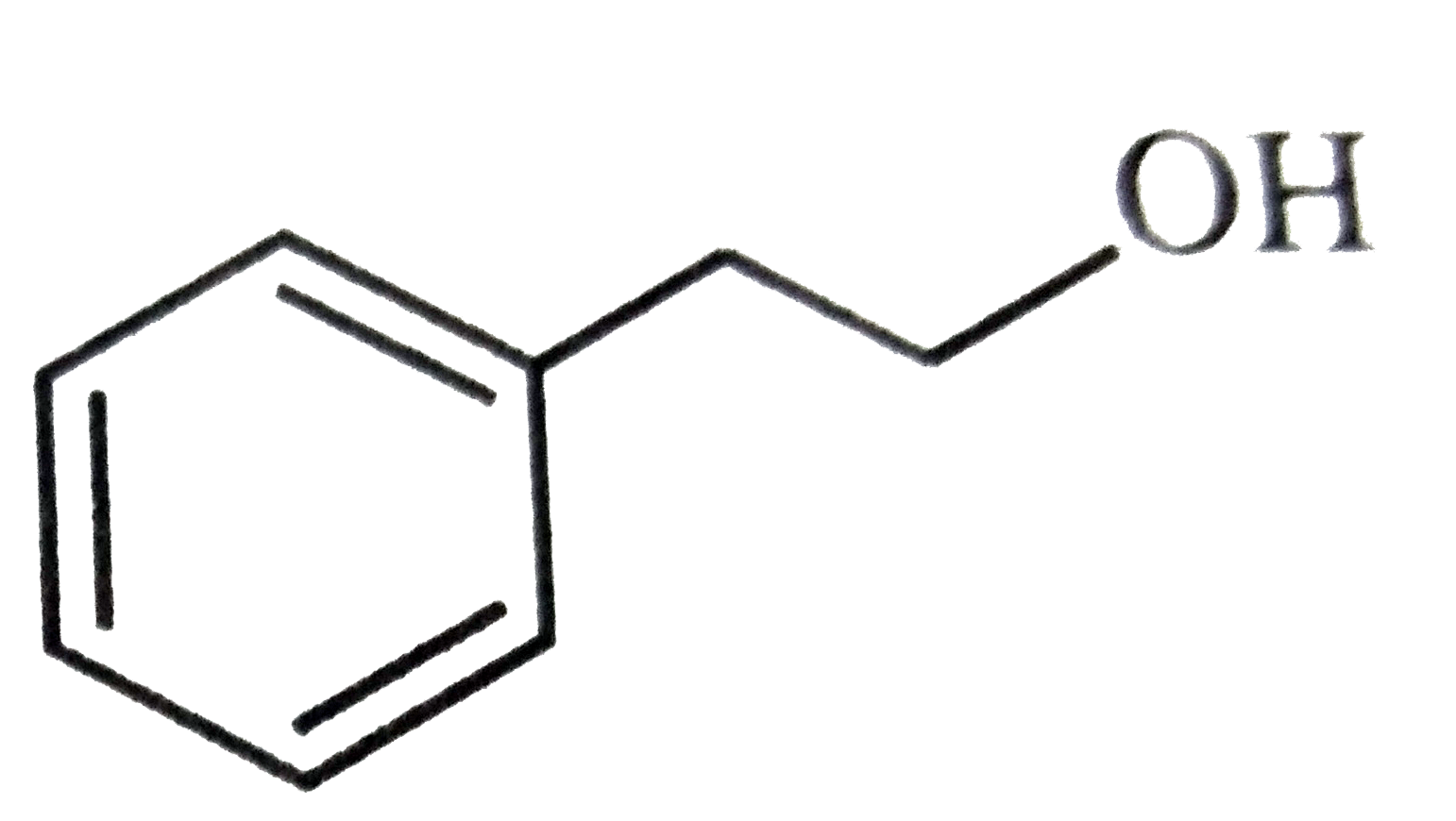
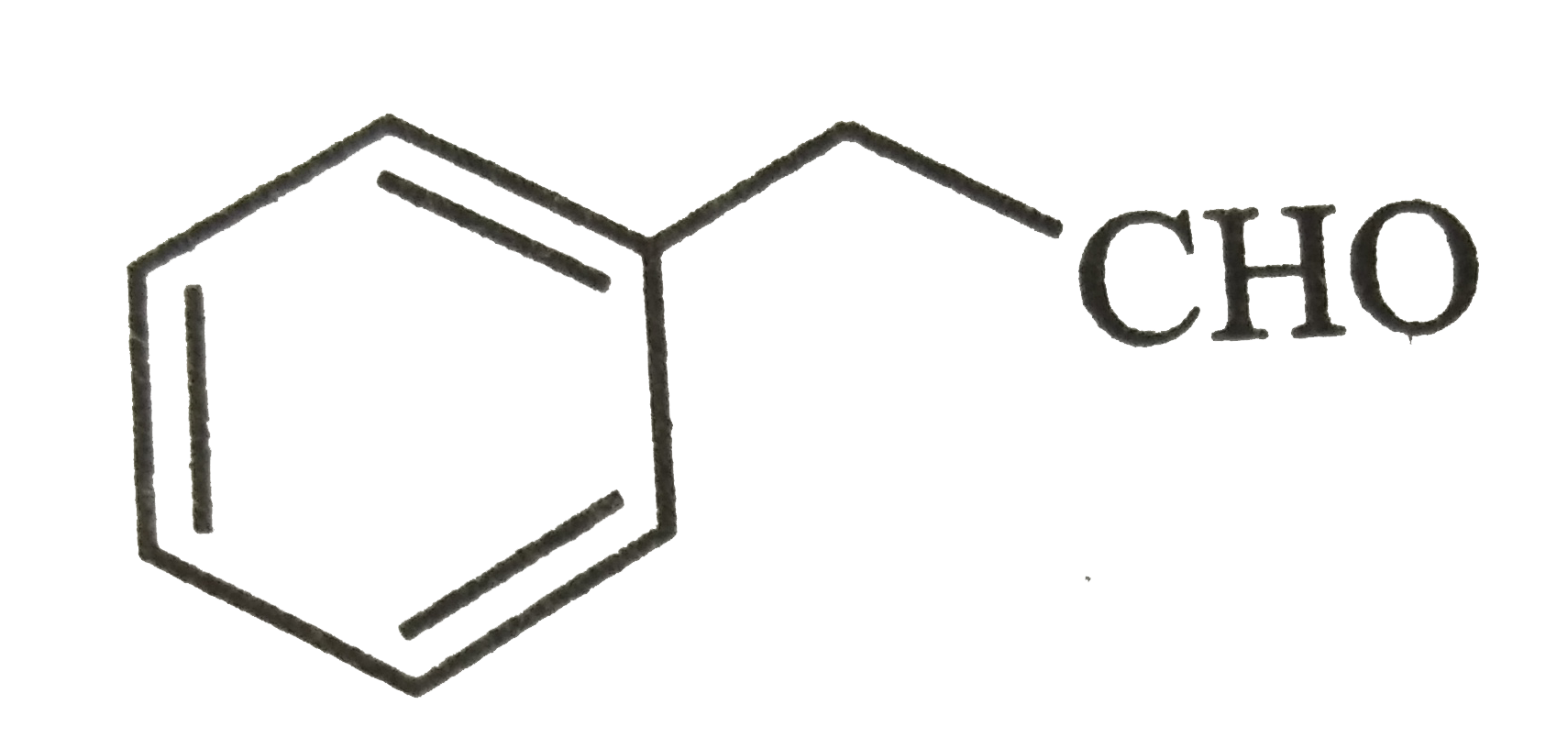
The correct Answer is:C
(a) to determine the structure of the compound with M.F. C8H6
(i) D.B.E. of C8H6=8(4−2)+6(1−2)2+1=6
(ii) Out of these six D.B.Es., fopupr may be due to the presence of a benzene ring.
the presence of a benzene ring.
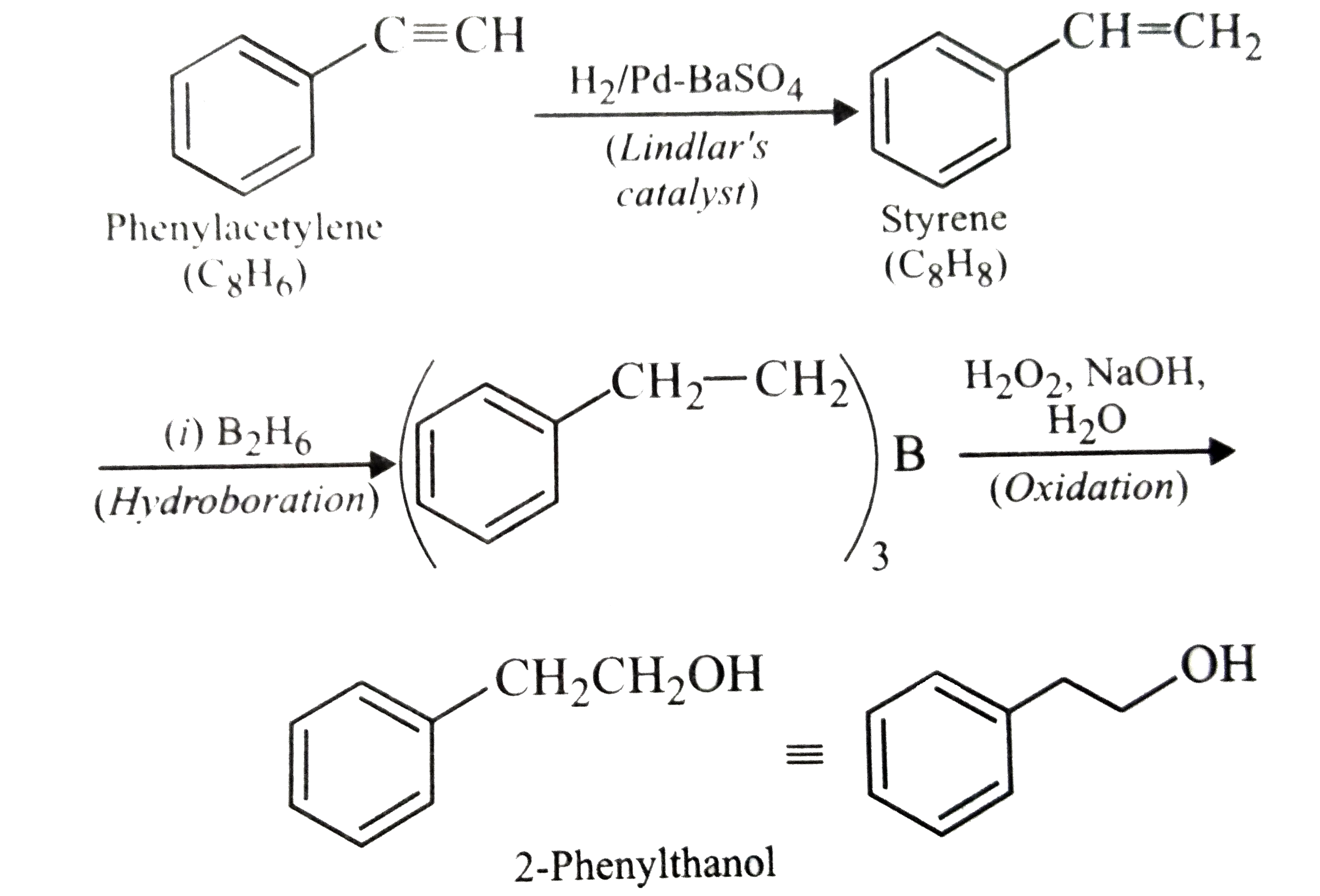
(iii) since the compound has to total of 8 carbons, six of which are due to the presence of a benzene ring, therefore, the side chain must contain two carbon atoms and two D.B.Es. the only possibility is that the sid echain contains an acetylenic group, i.e., −C≡CH and the compound with M.F. C8H6 must be phenylacetylene, C6H5C≡CH.
(b) To deduce the structure compound (X)
(i) Phenylacetylene (C8H6) on partial reduction with Lindlar's catalyst (H2/Pd−BaSO4) gives C8H8 which must be styrene (C6H5CH=CH2).
(ii) Styrene on hydroboration-oxidation gives compound (X), therefore, X must be 2-phenylethanol.
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBook:PRADEEPChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MATCHING TYPE QUESTIONS-IV)
Explore 4 Videos
- ALCOHOLS, PHENOLS AND ETHERSBook:PRADEEPChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MATRIX-MATCH TYPE QUESTIONS-V)
Explore 1 Video
- ALCOHOLS, PHENOLS AND ETHERSBook:PRADEEPChapter:ALCOHOLS, PHENOLS AND ETHERSExercise:COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER-II)
Explore 12 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:PRADEEPChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 29 Videos
Similar Questions
Three compound X, Y and Z were taken into three different laboratory vessels annd they are carried out by a chemist in his car. The car caught fire due to short circuit and the chemist came out of the car and noticed following observations:
Q. Compound X changes into white substnace along with liberation of neutral oxide and then white substnace decomposed into three products among which two are acidic oxides. among these oxides non-polar oxide can undergo polar cyclic polymer on cooling. the compound X will be:
View Solution
Compound 'Q' is:
View Solution
Knowledge Check
Test (Q). A compound 'X' was fused with Na metal and the extract gave a white precipitate with AgNO3. The Lassaigne's extract gave red colouration with neutral FeCl3. Test R while compound 'Y' when fused with Na metal and subsequent analysis on its Lassaigne's extract did not give any characteristic test. Test S while compound Y on fusion with fusion mixture (sodium carbonate+potassium nitrate) or Na2O2, followed by extraction, followed by addition of conc. HNO3 and ammonium molybdate gave a yellow precipitate
Q. Compound X containsANBSCN+SDPThree compound X, Y and Z were taken into three different laboratory vessels annd they are carried out by a chemist in his car. The car caught fire due to short circuit and the chemist came out of the car and noticed following observations:
Q. Compound X changes into white substnace along with liberation of neutral oxide and then white substnace decomposed into three products among which two are acidic oxides. among these oxides non-polar oxide can undergo polar cyclic polymer on cooling. the compound X will be:AMgSO4⋅7H2OBZnSO4⋅7H2OCCaSO4⋅2H2ODFeSO4⋅7H2O Compound 'Q' is: A
Compound 'Q' is: A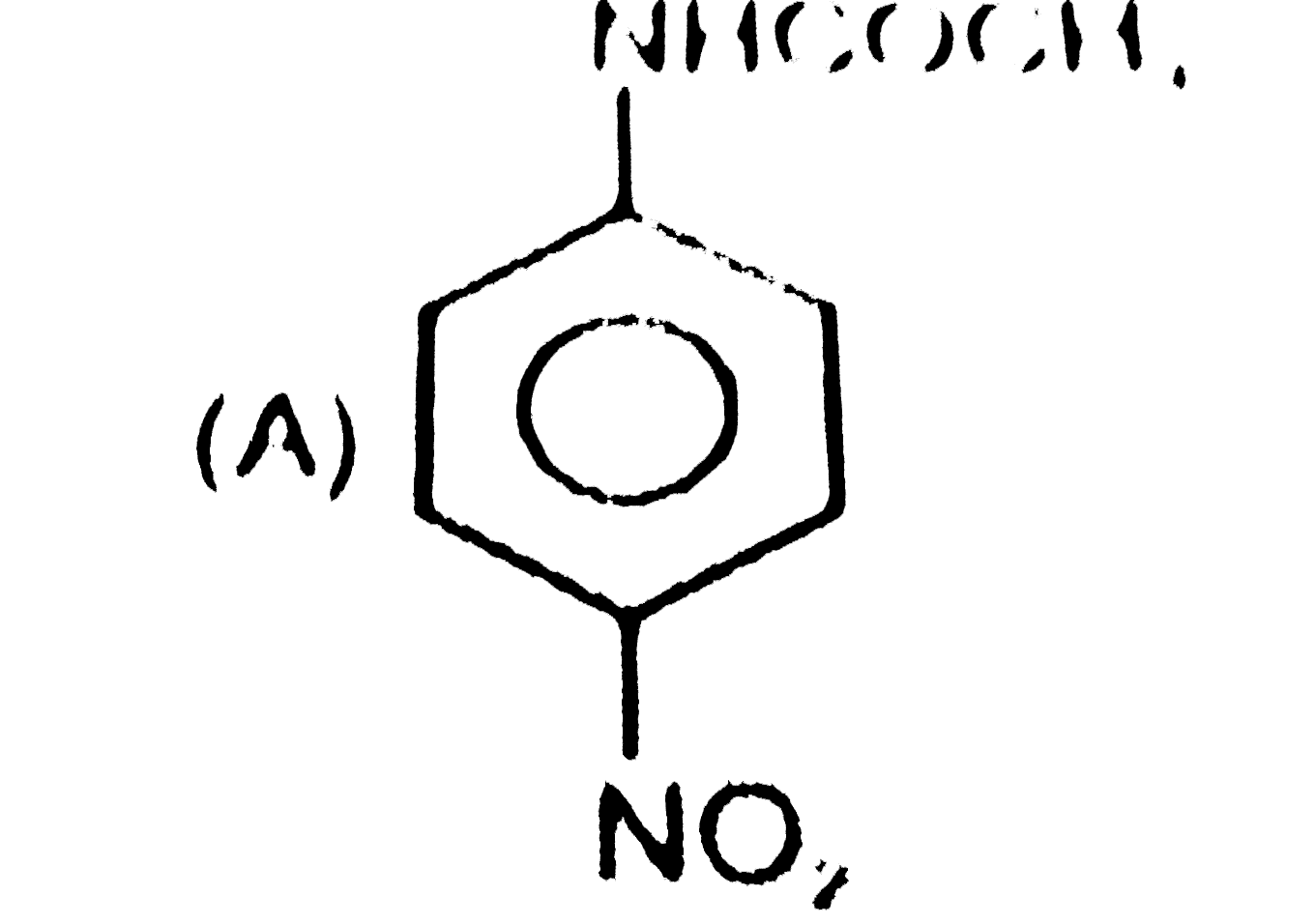 B
B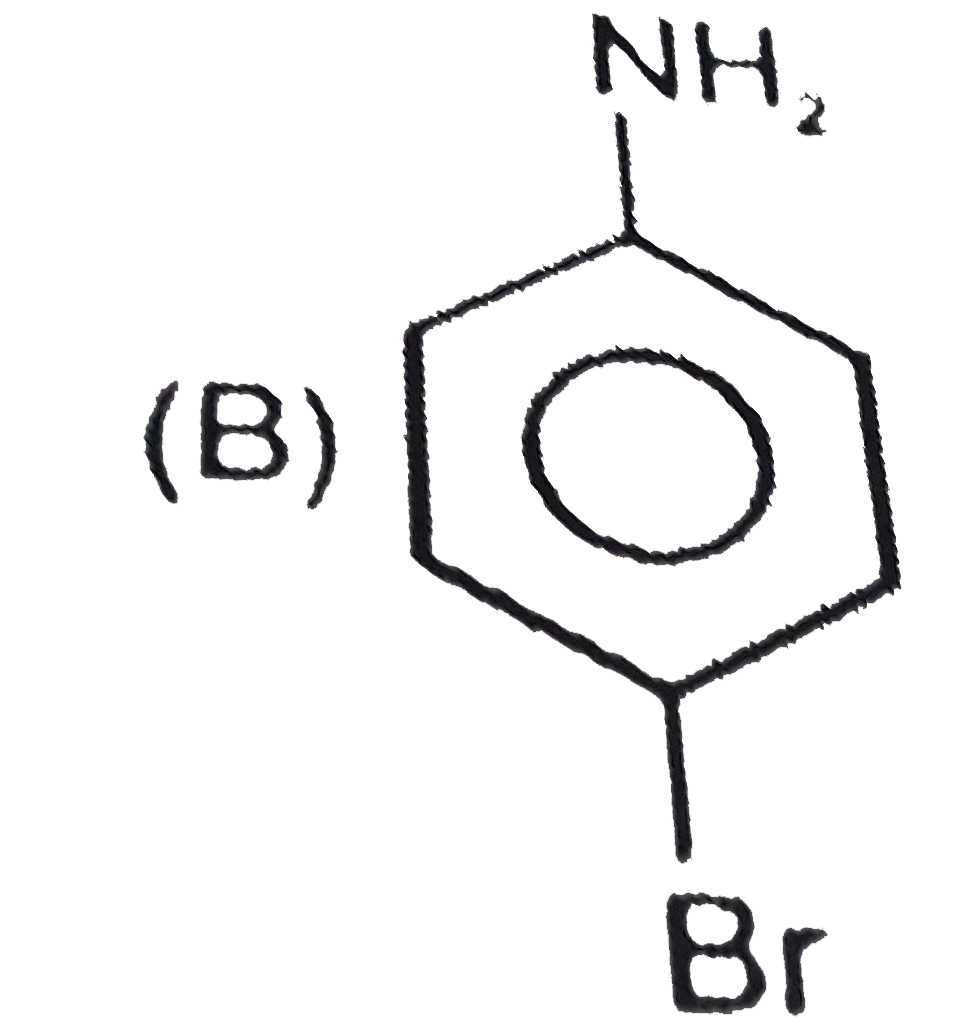 C
C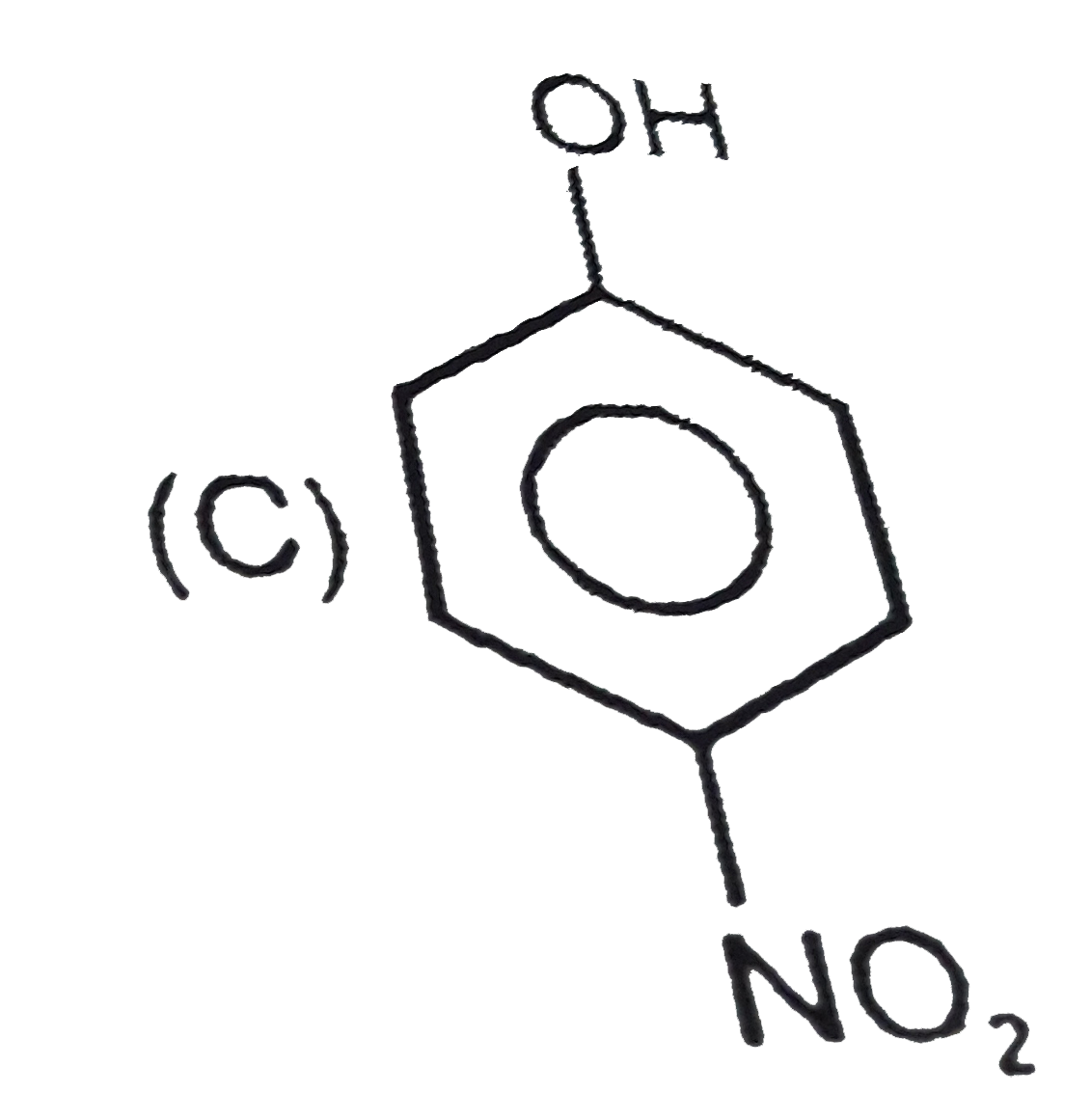 D
D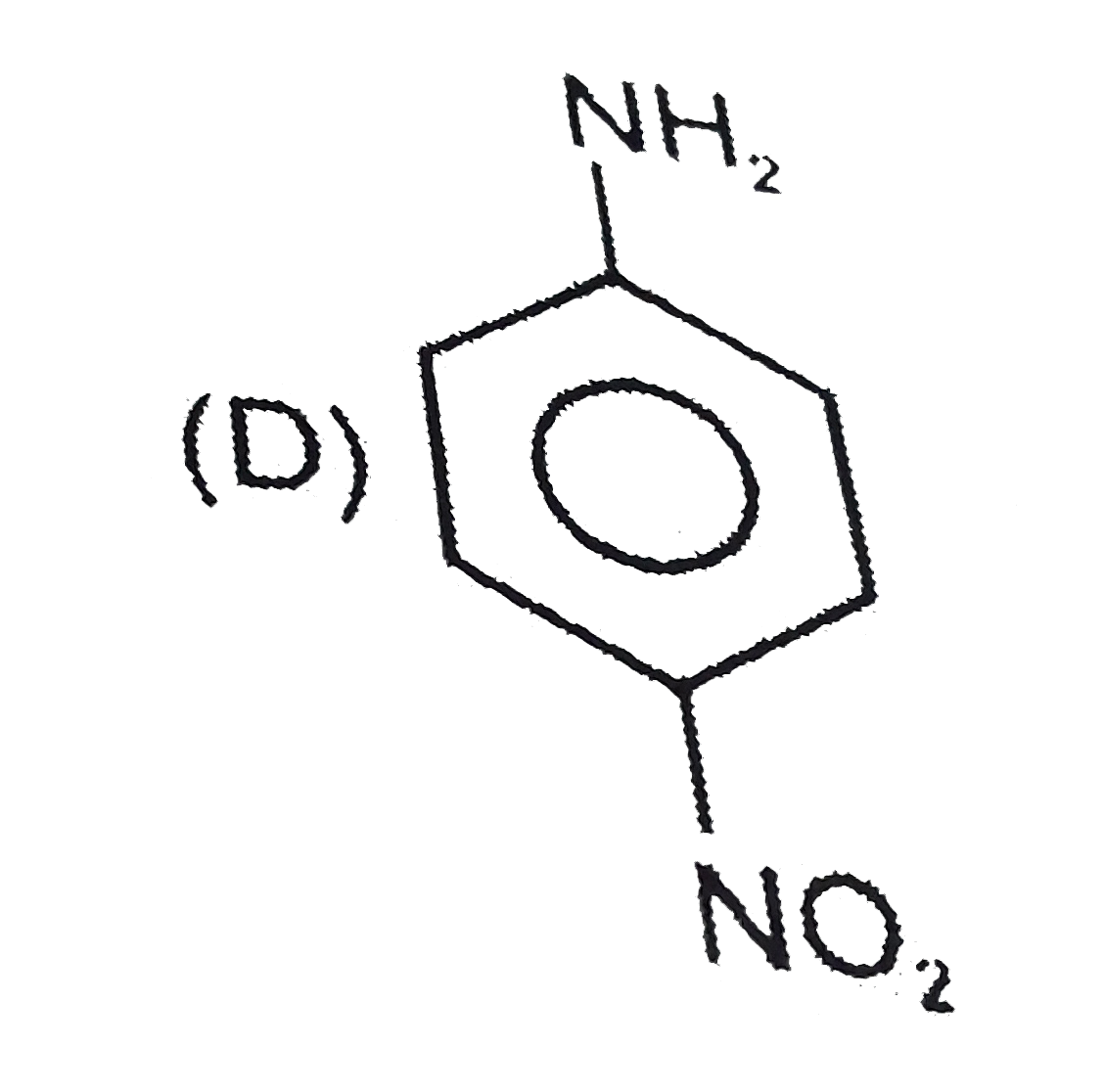
Compound 'Q' is :
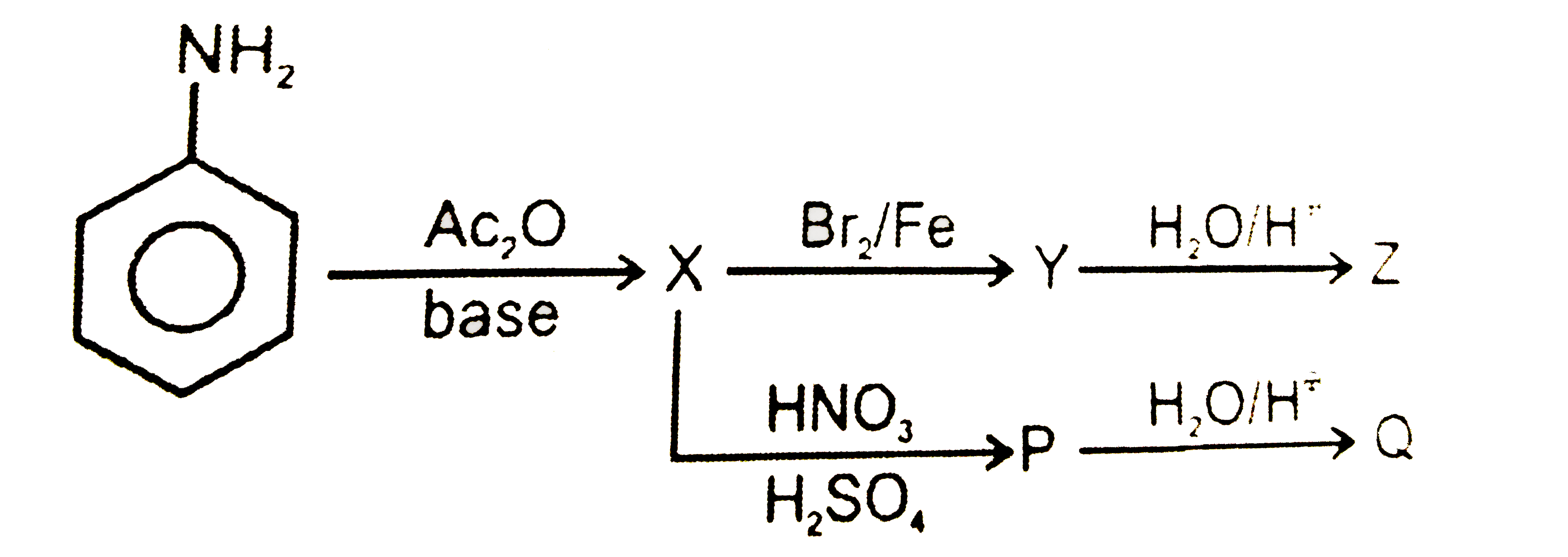
View SolutionCompound 'Q' is :

View SolutionTest (Q). A compound 'X' was fused with Na metal and the extract gave a white precipitate with AgNO3. The Lassaigne's extract gave red colouration with neutral FeCl3. Test R while compound 'Y' when fused with Na metal and subsequent analysis on its Lassaigne's extract did not give any characteristic test. Test S while compound Y on fusion with fusion mixture (sodium carbonate+potassium nitrate) or Na2O2, followed by extraction, followed by addition of conc. HNO3 and ammonium molybdate gave a yellow precipitate
Q. Compound X
View SolutionAn organic compound 'P (mol mass -75) on reduction gives compound 'Q' which yields compound 'R' when treated with nitrous acid. Compound R responds to iodofonn reaction. Compound Q is
View SolutionThe magnetic property, dipole moment, plane of symmetry, colour and absorption band cann be helpful in structure elucidation of complex compounds:
Q. Compound 'x' has molecular formula CrCl2Br⋅6H2O can show type of isomerism.
(i) hydrate isomerism
(ii) ionization isomerism
(iii) geometrical isomerism
(iv) optical isomerism
View Solution

