By a proper choice of reagent, both symmetrical and unsymmetrical ethers can by prepared by williamson synthesis which involves the reaction between an alkyl halide and an alkoxide ion. The reverse process involving the cleavage of etehrs to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at 373K.
Q. Benzyl ethyl ether reacts with HI to form
p-iodotoluene annd ethyl alcohol
benzyl alcohol and ethyl iodide
benzyl iodide ad ethyl alcohol
iodobenzene and ethyl alcohol
The correct Answer is:C
To solve the question regarding the reaction of benzyl ethyl ether with HI, we will follow these steps:
1. Identify the Structure of Benzyl Ethyl Ether:
- Benzyl ethyl ether can be represented as C6H5CH2-O-CH2CH3. It consists of a benzyl group (C6H5CH2) and an ethyl group (CH2CH3) connected by an ether bond (–O–).
2. Understand the Reaction Conditions:
- The ether will be cleaved by heating with hydroiodic acid (HI) at 373 K. This reaction involves the breaking of the ether bond to regenerate the alkyl halide and alcohol.
3. Cleavage Mechanism:
- When benzyl ethyl ether reacts with HI, the ether bond (C–O) is broken. The iodine (I) from HI will attach to one of the alkyl groups, while the hydroxyl (OH) group will be formed from the other.
4. Identify the Products:
- The benzyl group (C6H5CH2) will react with HI to form benzyl iodide (C6H5CH2I).
- The ethyl group (CH2CH3) will react with HI to form ethanol (CH3CH2OH).
5. Write the Overall Reaction:
- The overall reaction can be summarized as:
C6H5CH2-O-CH2CH3+HI→C6H5CH2I+CH3CH2OH
Final Answer:
Benzyl ethyl ether reacts with HI to form benzyl iodide and ethanol.
---
- Benzyl ethyl ether can be represented as C6H5CH2-O-CH2CH3. It consists of a benzyl group (C6H5CH2) and an ethyl group (CH2CH3) connected by an ether bond (–O–).
- The ether will be cleaved by heating with hydroiodic acid (HI) at 373 K. This reaction involves the breaking of the ether bond to regenerate the alkyl halide and alcohol.
- When benzyl ethyl ether reacts with HI, the ether bond (C–O) is broken. The iodine (I) from HI will attach to one of the alkyl groups, while the hydroxyl (OH) group will be formed from the other.
- The benzyl group (C6H5CH2) will react with HI to form benzyl iodide (C6H5CH2I).
- The ethyl group (CH2CH3) will react with HI to form ethanol (CH3CH2OH).
- The overall reaction can be summarized as:
C6H5CH2-O-CH2CH3+HI→C6H5CH2I+CH3CH2OH
Benzyl ethyl ether reacts with HI to form benzyl iodide and ethanol.
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBOOK - PRADEEPCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MATCHING TYPE QUESTIONS-IV)
4 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - PRADEEPCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MATRIX-MATCH TYPE QUESTIONS-V)
1 Video
- ALCOHOLS, PHENOLS AND ETHERSBOOK - PRADEEPCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER-II)
12 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - PRADEEPCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - IMPORTANT QUESTIONS FOR BOARD EXAMINATION
29 Videos
Similar Questions
Match the chemical conversion in List-I with the approprotae reagents in List-II and select the correct answer using the code given below this list-
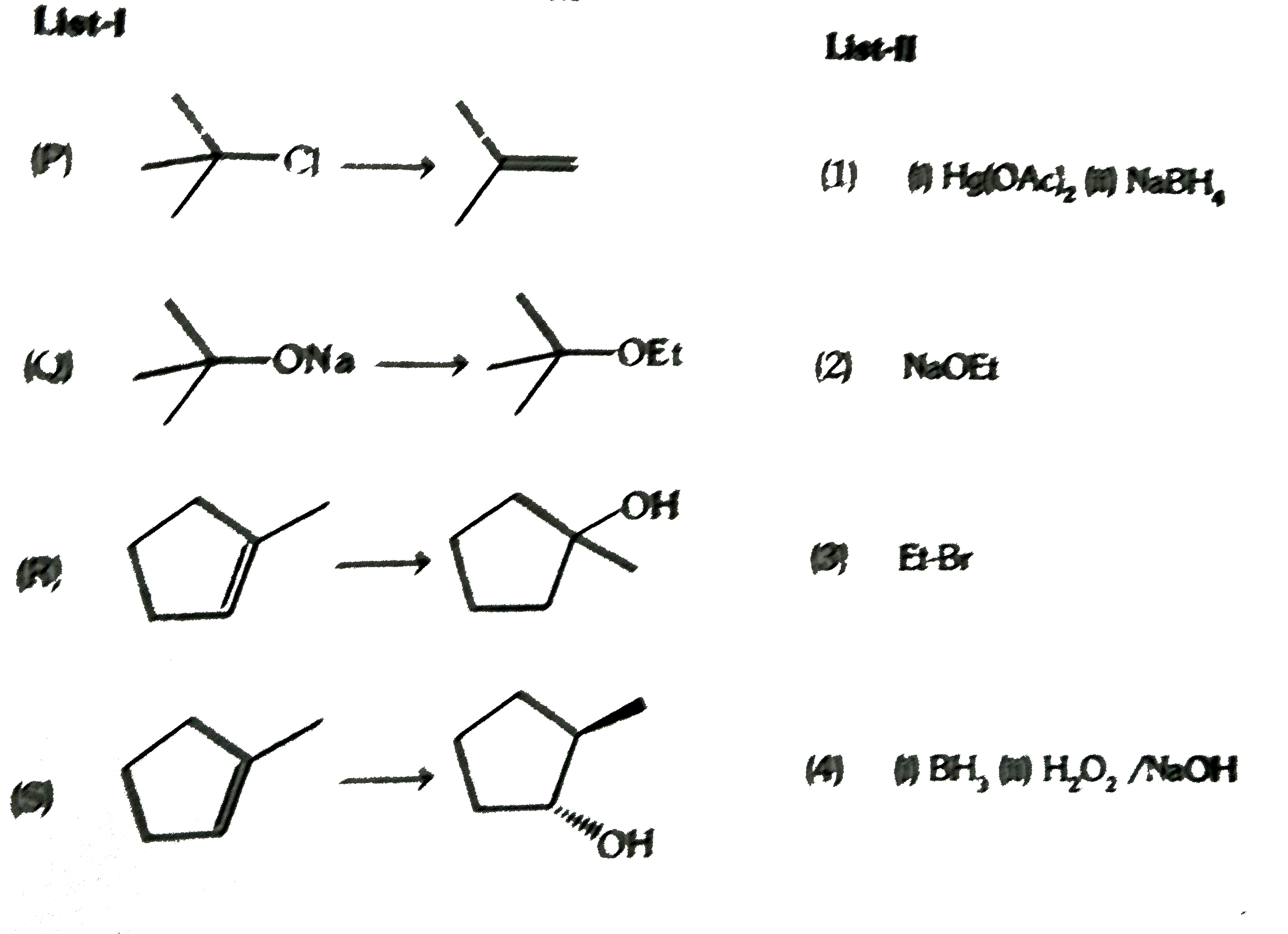 ,
,
View SolutionMatch the following columns

View SolutionThe correct combination of names for isomeric alcohols with molecular formula C4H10O is/are
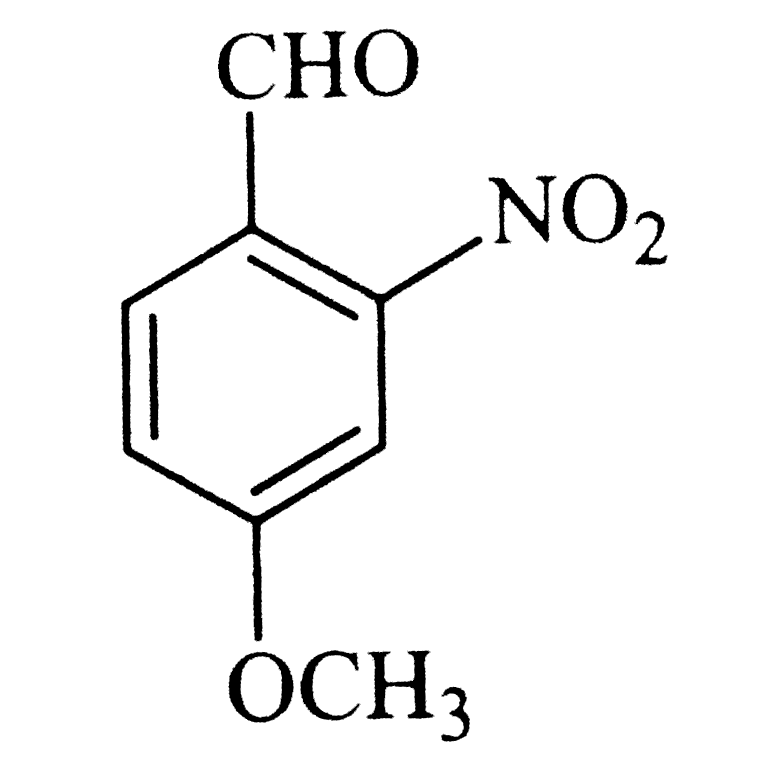
View SolutionGive the IUPAC name of the following compound
View SolutionEthers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-buty ether can't be prepared by this method. Explain
View SolutionEthers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-buty ether can't be prepared by this method. Explain
View Solutionयदि ऐल्किल हैलाइड तरश्यारी हो तो क्या एक ईथर के निर्माण के लिए विलियमसन संश्लेषण का उपयोग किया जा सकता है?
View SolutionEthers can be prepared by (1)heating alkyl halide with R-OH (2)boiling alkyl halide with alc.KOH (3)heating alkyl halide with sodium alkoxide (4)reacting alcohol with diazomethane
View SolutionBy a proper choice of reagent, both symmetrical and unsymmetrical ethers can by prepared by williamson synthesis which involves the reaction between an alkyl halide and an alkoxide ion. The reverse process involving the cleavage of etehrs to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at 373K.
Q. Allyl phenyl ether can be prepared by heating
View SolutionBy a proper choice of reagent, both symmetrical and unsymmetrical ethers can by prepared by williamson synthesis which involves the reaction between an alkyl halide and an alkoxide ion. The reverse process involving the cleavage of etehrs to give back the original alkyl halide and the alcohol can be carried out by heating the ether with HI at 373K. Q. Which of the following ethers is not cleaved by HI?
View SolutionName the pair of alkyl halide and alkoxide for the preparation of ethyl tert-butyl ether.
View SolutionEther can be obtained by reacting alkoxide with an alkyl halide. This is __________
View SolutionEthers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tertiary ether can't be prepared by this method. Explain.
View SolutionEthers can be prepared by Williamson synthesis which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can't be prepared by this method. Explain.
View SolutionAn alkyl halide reacts with alkoxide to form ether. This is known as ________ synthesis.
View Solution

