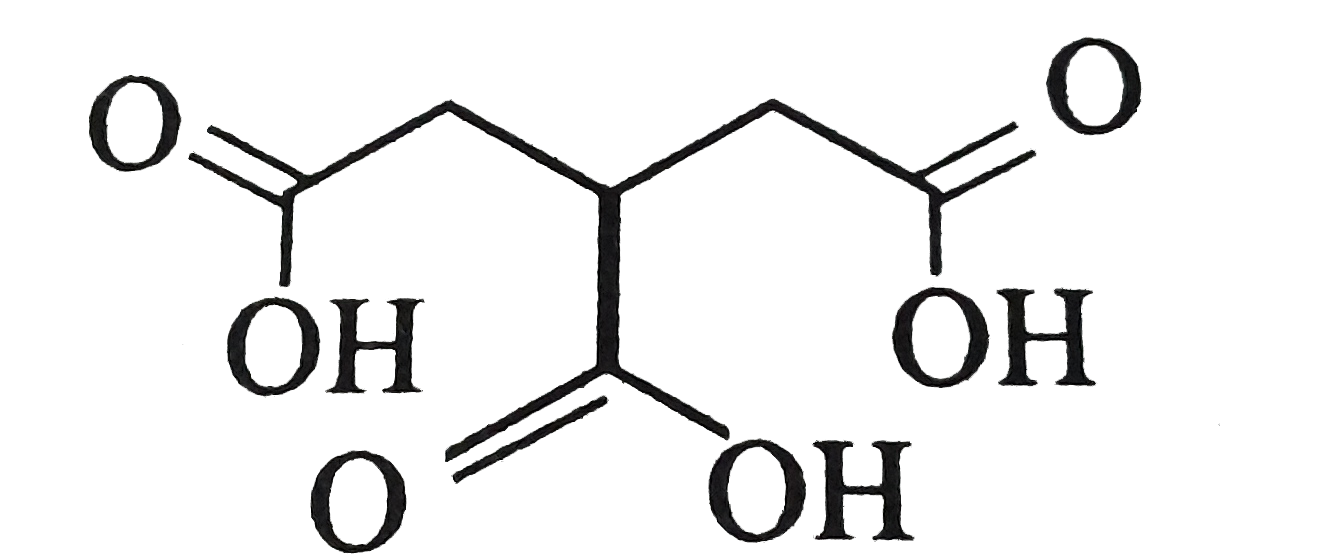
Write the IUPAC name of the product formed when nitrobenzene is reduced using tin and concentrated hydrochloric acid.
To determine the IUPAC name of the product formed when nitrobenzene is reduced using tin and concentrated hydrochloric acid, we can follow these steps:
Step 1: Identify the Reactant
The starting compound is nitrobenzene, which has the chemical formula C6H5NO2. It consists of a benzene ring with a nitro group (–NO2) attached to it.
Step 2: Understand the Reduction Reaction
When nitrobenzene undergoes reduction, it loses the nitro group and gains an amino group (–NH2). The reducing agent in this reaction is tin (Sn) in the presence of concentrated hydrochloric acid (HCl).
Step 3: Determine the Product
The reduction of nitrobenzene leads to the formation of aniline, which has the chemical formula C6H5NH2. Aniline is an aromatic amine where the amino group is directly attached to the benzene ring.
Step 4: Assign the IUPAC Name
The IUPAC name for aniline is simply "aniline." However, if we were to provide a systematic IUPAC name, it would be "benzenamine," indicating that the amino group is attached to a benzene ring.
Final Answer
The IUPAC name of the product formed when nitrobenzene is reduced using tin and concentrated hydrochloric acid is benzenamine.
---
The starting compound is nitrobenzene, which has the chemical formula C6H5NO2. It consists of a benzene ring with a nitro group (–NO2) attached to it.
When nitrobenzene undergoes reduction, it loses the nitro group and gains an amino group (–NH2). The reducing agent in this reaction is tin (Sn) in the presence of concentrated hydrochloric acid (HCl).
The reduction of nitrobenzene leads to the formation of aniline, which has the chemical formula C6H5NH2. Aniline is an aromatic amine where the amino group is directly attached to the benzene ring.
The IUPAC name for aniline is simply "aniline." However, if we were to provide a systematic IUPAC name, it would be "benzenamine," indicating that the amino group is attached to a benzene ring.
The IUPAC name of the product formed when nitrobenzene is reduced using tin and concentrated hydrochloric acid is benzenamine.
Similar Questions
Knowledge Check
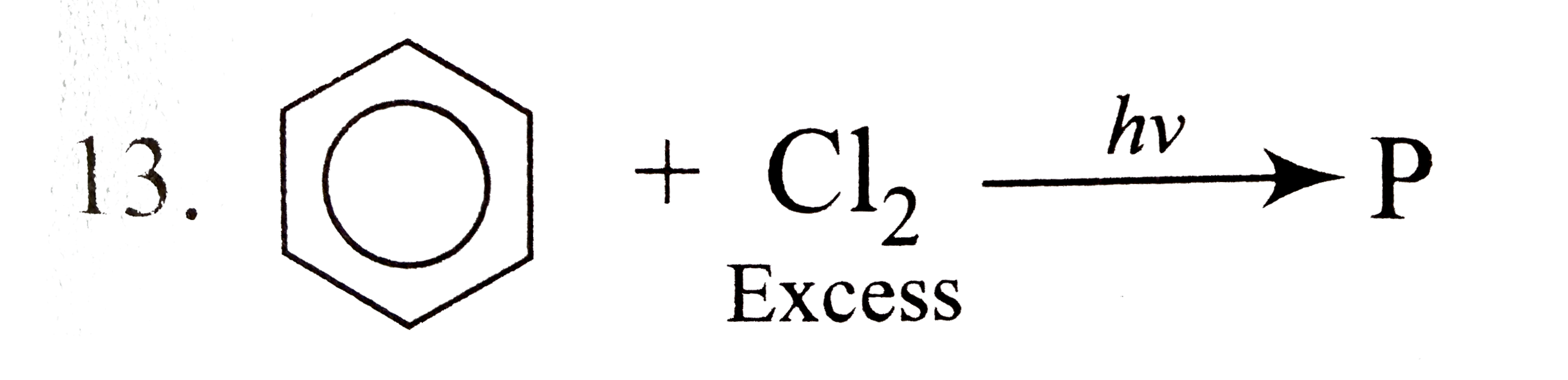
The IUPAC name of the product P isABenzene hexachlorideBHexachlorobenzeneCHexachlorocyclohexanaD1,2,3,4,5,6-hexachlorocyclohexaneWhat is formed, when nitrobenzene is reduced using zinc and alkali?
APhenolBAnilineCNitrosobenzeneDHydrazonbenzeneHydrobenzene is formed when nitrobenzene is reduced with
AZn/HClBZn/NaOHCZn/NaOH−CH3OHDZn/NH4Cl.