Hydrobenzene is formed when nitrobenzene is reduced with
A
Zn/HCl
B
Zn/NaOH
C
Zn/NaOH−CH3OH
D
Zn/NH4Cl.
Text Solution
The correct Answer is:B
C6H5NO2Zn(NaOH)−−−−−−−→C6H5−NH−NHC6H5
|
Similar Questions
Knowledge Check
When reduction of nitrobenzene is carride out in alkaline medium it forms different products depending upon the nature of the reducing agent Azoxybenzene is obtained when nitrobenzene is reduced with
ALiAIH4 in etherBNa3AsO3/NaOHCZn/NaOH,CH3OHDZn/NaOHThe structure of the compound formed, when nitrobenzene is reduced by lithium aluminium hydride (LiAlH4) is
A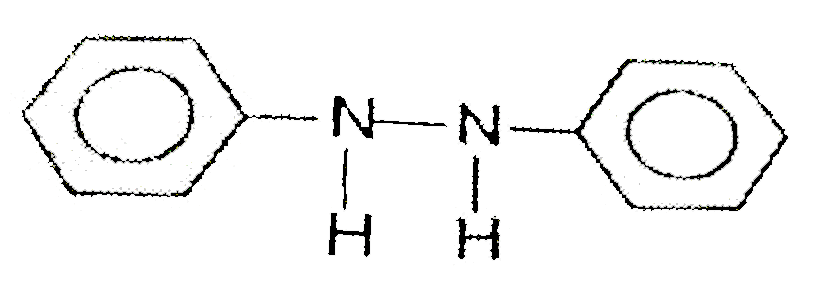 B
B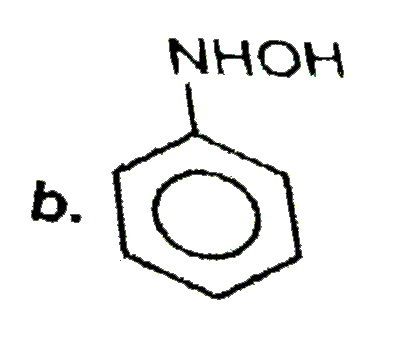 C
C D
D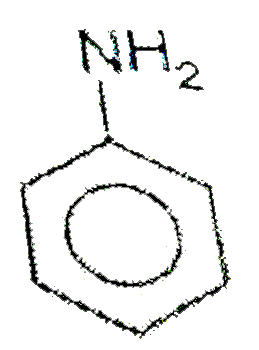
What is formed, when nitrobenzene is reduced using zinc and alkali?
APhenolBAnilineCNitrosobenzeneDHydrazonbenzene
The final product formed when acetaldehyde is reduced with sodium and alcohol is
View SolutionWhat are the products formed when phenol and nitrobenzene are treated separately with a mixture of concentrated sulphuric acid and concentrated nitric acid ?
View SolutionWhile is the product when nitrobenzene is treated with zinic dust and ammoniun chloride
View SolutionWhich of the following is formed when picric acid is reduced with sodium sulphide?
View SolutionThe final product formed when acetaldehude is reduced with sodium and alcohol is
View Solution

