Which of the following will result in zero overlap if molecular axis is x−axis ?
1s−2px
2s−2pz
2px−2px
1s−2py
The correct Answer is:B, D
If direction of approching orbital is not proper then it is known as zero overtapping.
In 2s−2pz and 1s−2py direction of approching orbital is not proper.
Similar Questions
Knowledge Check
Which of the following is a zero overlap which leads to non-bonding?
A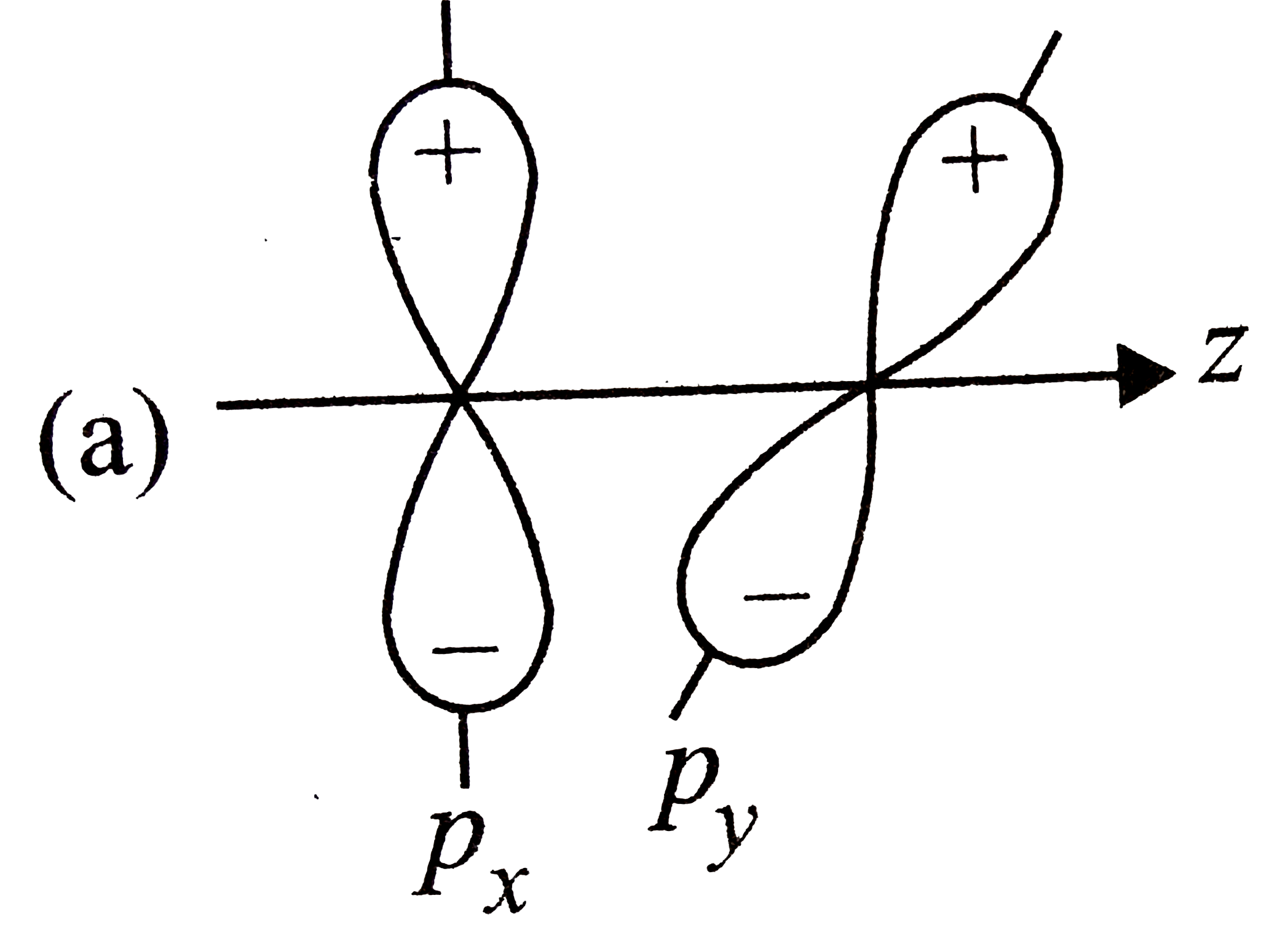 B
B C
C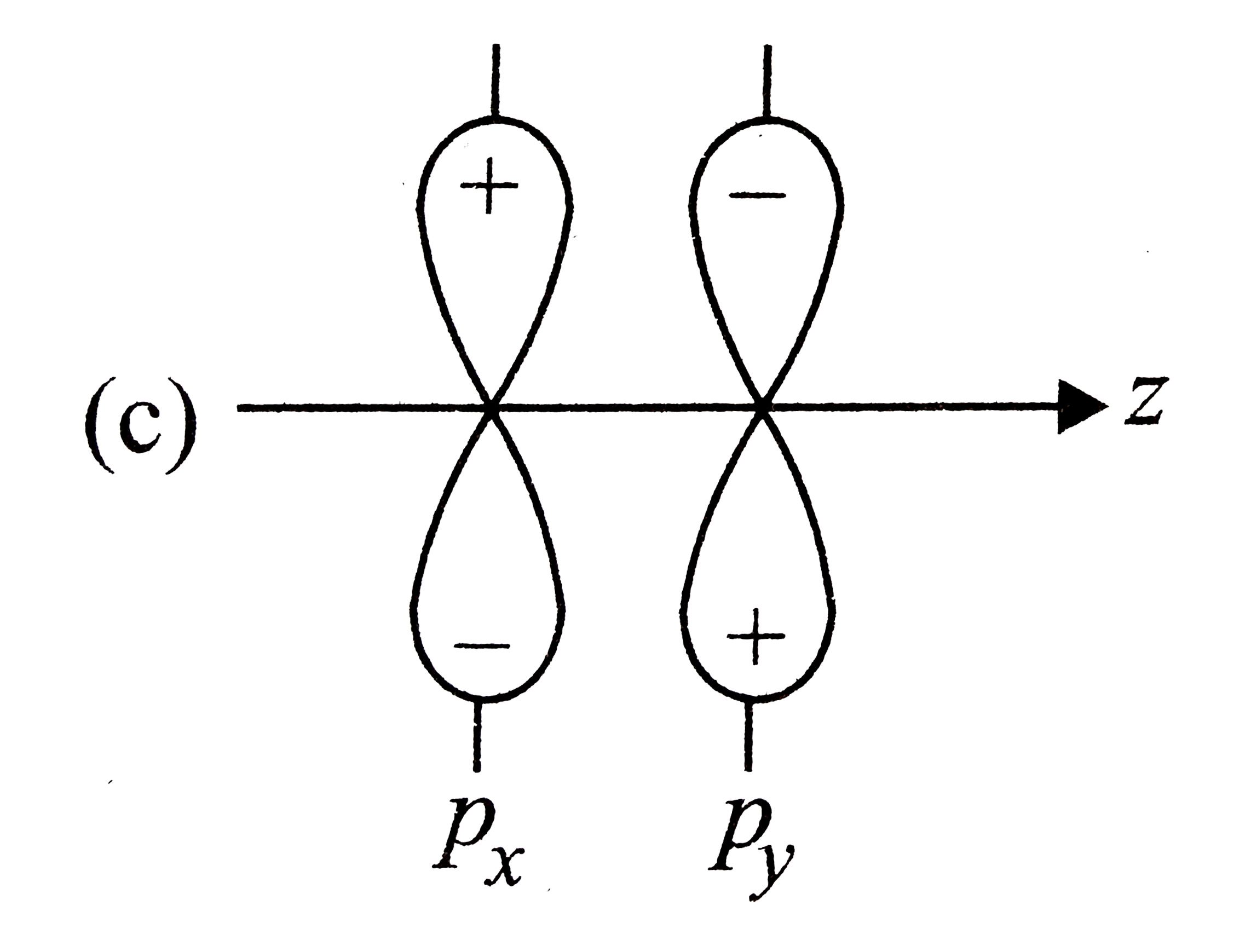 DAll
DAllWhich of the following would result in the formation of strongest π- bond if the molecular axis is X-axis?
A2px+2pxB3py+3pyC3py+3dxyD3px+3dxyWhich of the following equations represents a line parallel to x axis
A2x+3=0B2y+2=0C2x+3y=0D2x-3y=0
Which of the following representation represent π∗ molecular orbital , (z is considered as internuclear axis)
View SolutionMolecular orbital theory is based on linear combination of atomic orbitals (LCAO). According to LCAO when respective atomic orbitals of the atoms interact, they undergo constructive and destructive interference giving rise to two types of molecular orbitals, that is, bonding and antibonding molecular orbitals.
If z is the molecular axis then which of the following overlapping results in non-bonding molecular orbital?
View SolutionWhich of the following combination of orbitals do / does not form bond (if x-axis is internuclear axis) ?
View SolutionMolecular axis is Z axis , then which of the following combination of orbitals will result in formation of σ molecular orbitals ?
View SolutionWhich of the following overlaps gives a σ bond with x as internuclear axis?
View Solution
