HCOONa350∘−−→ΔGas(X)+Rese(Y)CoCl3−−−→Complex(Z)
Complex (Z) is:
A
an outer orbital complex
B
optically active
C
oxidised when exposed to air
D
paramagnetic
Text Solution
The correct Answer is:D
HCOONa350∘C−−−→ΔH2(X)+NaC2O4(Y)CoCl3−−−→Na3[Co(C2O4](Z)
(i) Gas(X)→H2
-It is diamagnetic
-B.O.=1
-It acts as π-acid (into σ∗1s)
(ii) Na3[Co(C2O4]
[M(∀)3]
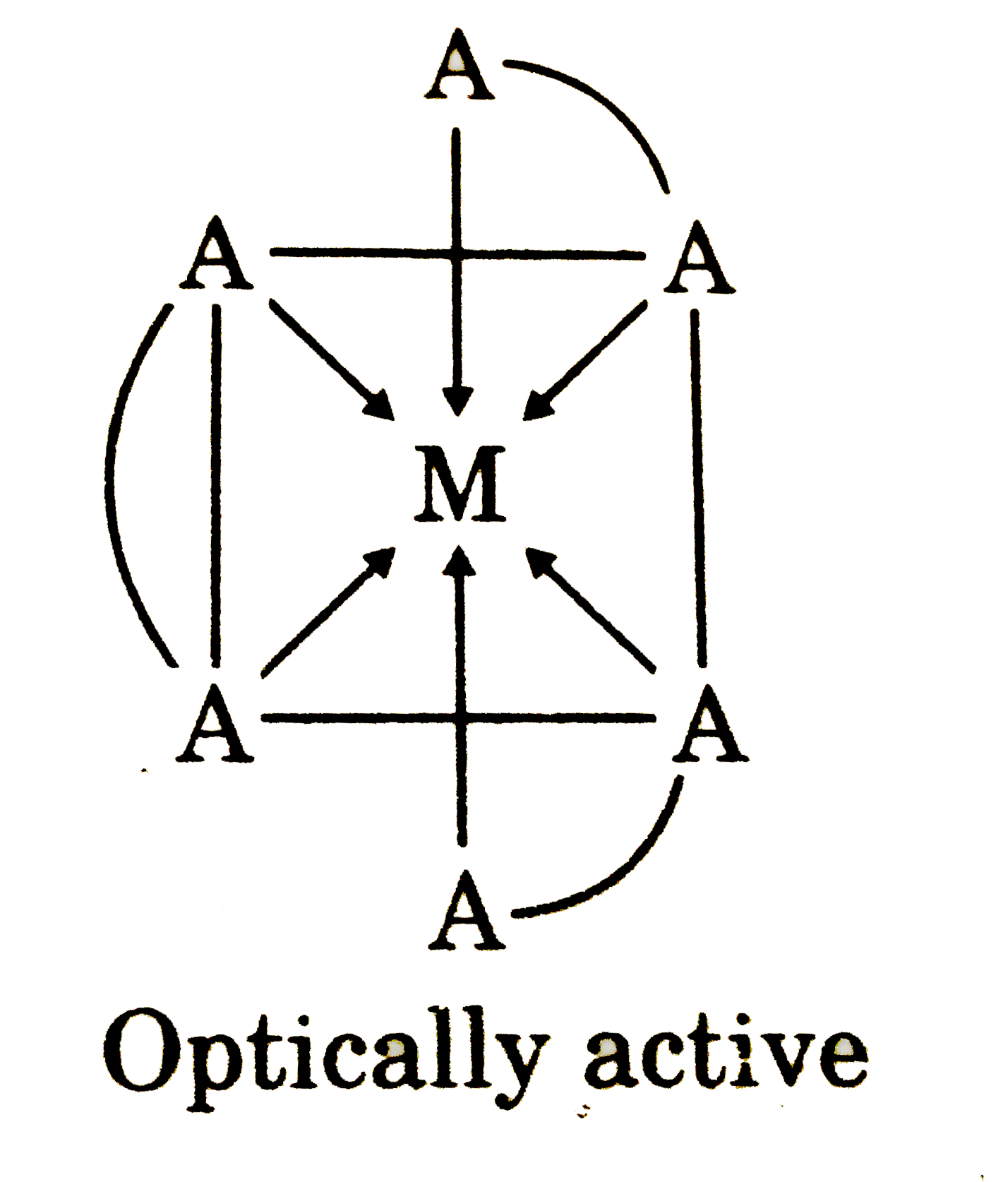
Optically active
|
Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 10
3 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 11
5 Videos
- COORDINATION COMPOUNDSBOOK - OP TANDONCHAPTER - COORDINATION COMPOUNDSEXERCISE - COMPREHENSION 8
3 Videos
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS BOOK - OP TANDONCHAPTER - CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS EXERCISE - INTEGER
2 Videos
- ELECTROCHEMISTRYBOOK - OP TANDONCHAPTER - ELECTROCHEMISTRYEXERCISE - Matrix-Matching Type Question
3 Videos
Similar Questions
Knowledge Check
HCOONa350∘−−→ΔGas(X)+Rese(Y)CoCl3−−−→Complex(Z)
Choose the correct statement regarding (X).AIt is paramagneticBIts bond order is oneCIt can't act as π−acDIt is a powerful oxidising agent.HCOONa350∘−−→ΔGas(X)+Rese(Y)CoCl3−−−→Complex(Z)
Choose the correct statement regarding (X).AIt is paramagneticBIts bond order is oneCIt can't act as π−acDIt is a powerful oxidising agent.R−O∣∣C−ClNH3−−→NH4Cl+(X)P4O10−−−→Δ(Y)(i)DIBAL−H−−−−−−−−−→(ii)H2O(Z)
The compound (Z) isAR-COOHBR−CH2−OHCR−C≡NDRCHO
Colourless salt (X)Δ−−→(Y)Cu2+,Δ−−−−−→ coloured bead (Z). (X) can be
View SolutionNiCl2KCN−−→HCNComplex A
NiCl2KCl−−−→excessComplex B
A and B complexes have the co-ordination number 4.
The IUPAC name of complexes A and B are respectively :
View SolutionNiCl2KCN−−→HCNComplex A
NiCl2KCN−−−→excessComplex B
A and B complexes have the co-ordination number 4.
The IUPAC name of complexes A and B are respectively :
View SolutionNiCl2KCN−−→HCNComplex A
NiCl2KCN−−−→excessComplex B
A and B complexes have the co-ordination number 4.
The hybridization of both complexes are :
View SolutionXSn/HCl−−−−→NaNO2−−−−→HClZCuCN−−−→Δ(P)H3O−−→Ph−COOH correct options is/are:
View Solution

