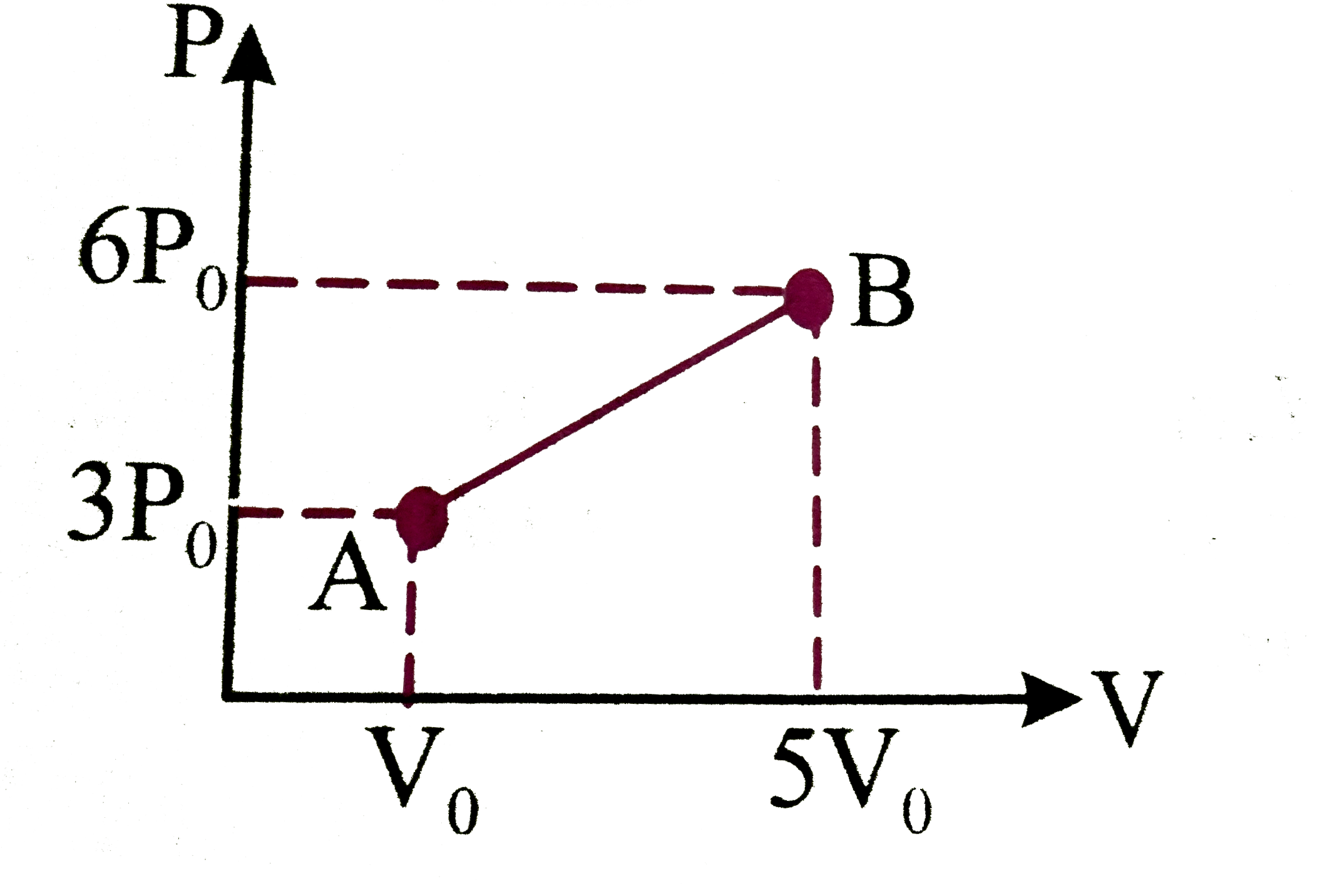
One mole of a monoatomic gas undergoes a linear process A→B in P−v diagram. The volume of the gas when the process turns from an endothermic to an exothermic one is
A
58v0
B
v02
C
8v015
D
3v05
Text Solution
The correct Answer is:A
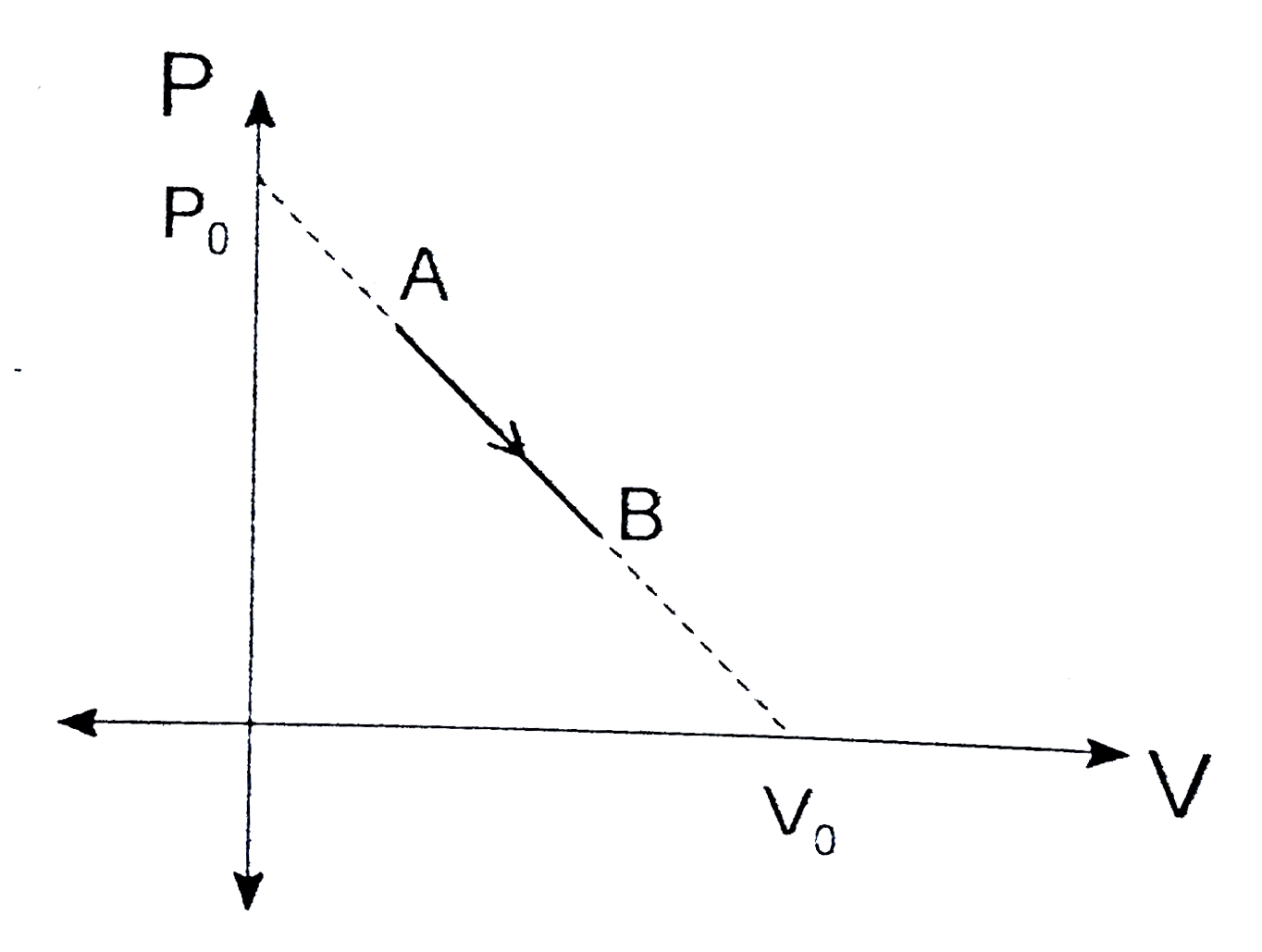
the point where adiabatic curve touch the line AB the nature of process will change from endo thermic to exothermic.
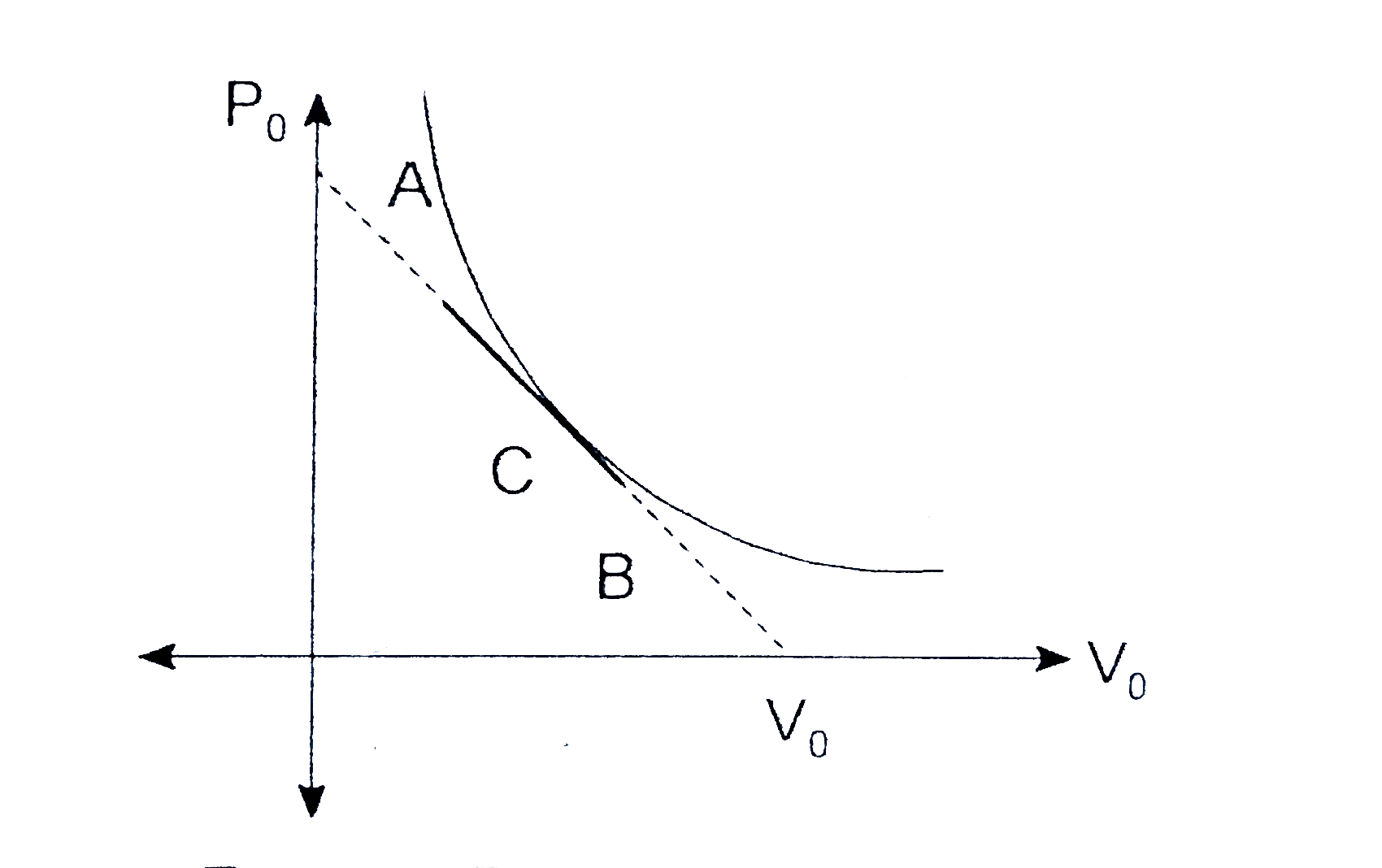
−P0v0=−γPV
−m=−γ(−mv+P0)Vm=+P0V0
mv(1+γ)=γP0 V=γP0m(1+γ)=58V0
|
Similar Questions
Knowledge Check
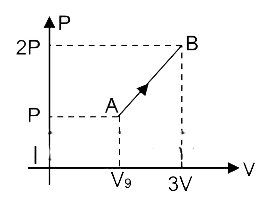
One mole of a monoatomic ideal gas undergoes process AB in given P – V diagram then average specific heat for this process is:
 A21R10B18R10C9R10D13R10
A21R10B18R10C9R10D13R10Two moles of an ideal monoatomic gas undergoes a cyclic process ABCA as shown in V-T diagram below:

Heat supplied to the gas during the process AB is :A1200Rln2B1800 RC1200 RDZeroOne mole of an ideal monoatomic gas undergoes a process as shown in the figure. Find the molar specific heat of the gas in the process.
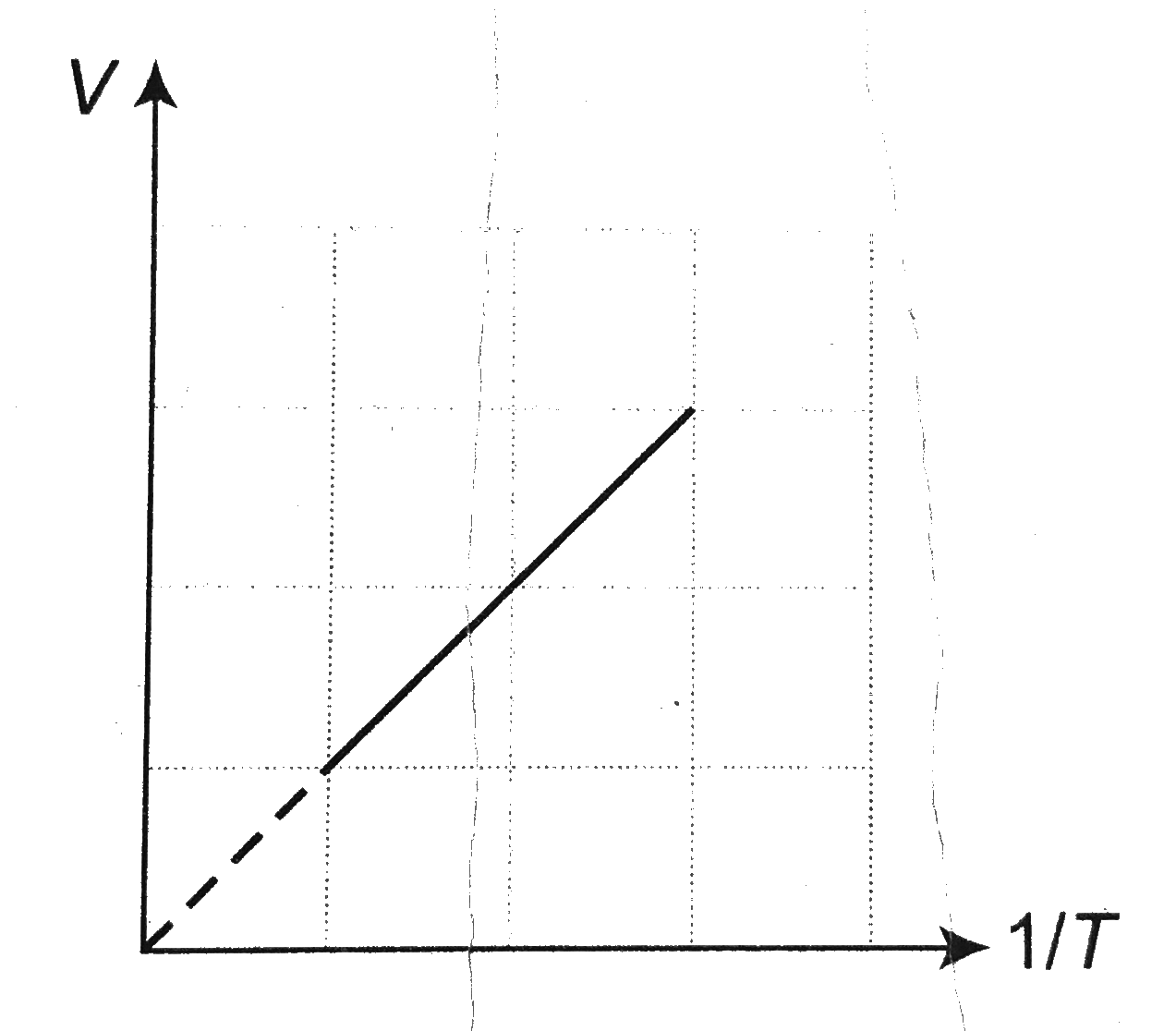
R is a gas constant.A3R4BR2CR3D2R3
One mole of monatomic ideal gas undergoes the process A→B , as in the given P−V diagram . The specific heat for this process is
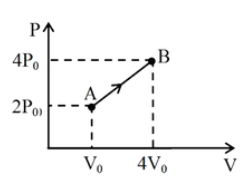
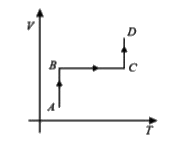
View SolutionOne mole of monoatomic gas undergoes a process as shown in the P-T diagram below. Choose the corresponding V – T diagram.
View SolutionOne mole of an ideal monoatomic gas undergoes a described by the equations PV3= constant The heat the gas during this process is :
View SolutionTwo moles of an ideal monoatomic gas undergoes a cyclic process ABCA as shown in V-T diagram below:

Work done by the gas during the entire cycle is :
View SolutionOne mole of an ideal monoatomic gas undergoes a cyclic process as shown in figure. Then the change in the energy in expanding the gas from a to c along the path abc is:
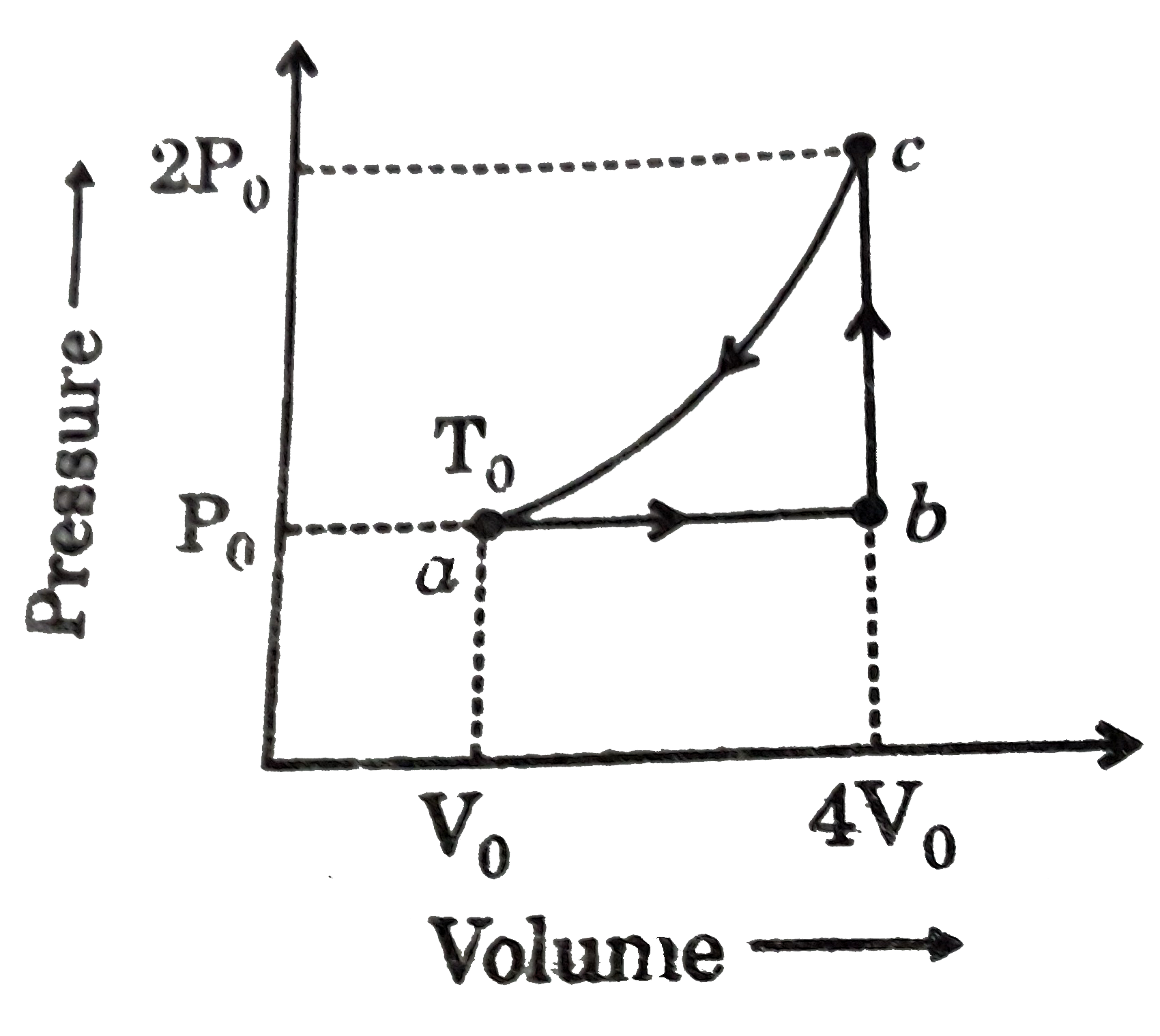
View Solution