Between Na+ and Ag+ which is stronger Lewis acid and why?
Video Solution
Text Solution
The correct Answer is:NA
|
Answer
Step by step video, text & image solution for Between Na^(+) and Ag^(+) which is stronger Lewis acid and why? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- CHEMICAL BONDINGBook:IIT-JEE PREVIOUS YEAR (CHEMISTRY)Chapter:CHEMICAL BONDINGExercise:MATCH THE COLUMNS
Explore 1 Video
- CHEMICAL BONDINGBook:IIT-JEE PREVIOUS YEAR (CHEMISTRY)Chapter:CHEMICAL BONDINGExercise:INTEGER_TYPE
Explore 1 Video
- CHEMICAL AND IONIC EQUILIBRIUMBook:IIT-JEE PREVIOUS YEAR (CHEMISTRY)Chapter:CHEMICAL AND IONIC EQUILIBRIUMExercise:INTEGER_TYPE
Explore 1 Video
- CHEMICAL KINETICSBook:IIT-JEE PREVIOUS YEAR (CHEMISTRY)Chapter:CHEMICAL KINETICSExercise:JEE Main And Advanced
Explore 66 Videos
Similar Questions
Knowledge Check
Which is the strongest Lewis acid?
ABF3BBCl3CBBr3DBl3.Which acid is stronger ?
AHA(pKa=4.7)BHB(pKa=3)CHC(pKa=2)DHD(pKa=2.7)Which one is Lewis acid
ACl−BAg+CC2H5OHDS2−
Between Na+ and Ag+ , which is stronger Lewis acid and why?
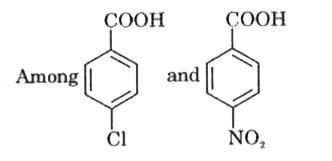
View SolutionExplain which is a stronger acid:
 .
.
View SolutionState whether the statement is true or false.BF3 is stronger Lewis acid than BCl3.
View SolutionWhich one is a Lewis acid
View SolutionWhich is the Lewis acid among following:
View Solution