Between Na+ and Ag+, which is a stronger Lewis acid and why?
Text Solution
Ag+ because Ag+ has pseudo noble gas configuration which makes it more polarising and therefore stronger acid.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
Which is the strongest Lewis acid?
ABF3BBCl3CBBr3DBl3.Which acid is stronger ?
AHA(pKa=4.7)BHB(pKa=3)CHC(pKa=2)DHD(pKa=2.7)Which is the Lewis acid among following:
ACl−B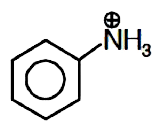 CSiCl4DCH3COOH
CSiCl4DCH3COOH
Between Na+ and Ag+ which is stronger Lewis acid and why?
View SolutionBetween Na+ and Ag+ , which is stronger Lewis acid and why?
View SolutionAmong
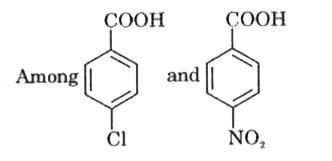
which is stronger acid and why ?
View SolutionExplain which is a stronger acid:
 .
.
View SolutionState whether the statement is true or false.BF3 is stronger Lewis acid than BCl3.
View Solution

