In the anion HCOO− , the carbon-oxygen bonds are found to be of equal length. This is due to :
The anion is obtained by the removal of a proton from the acid molecule.
Electronic orbitals of carbon atoms are hybridised.
The C=O bond is weaker than C−O bond.
The anion HCOO− has two equally stable resonating structures.
The correct Answer is:4
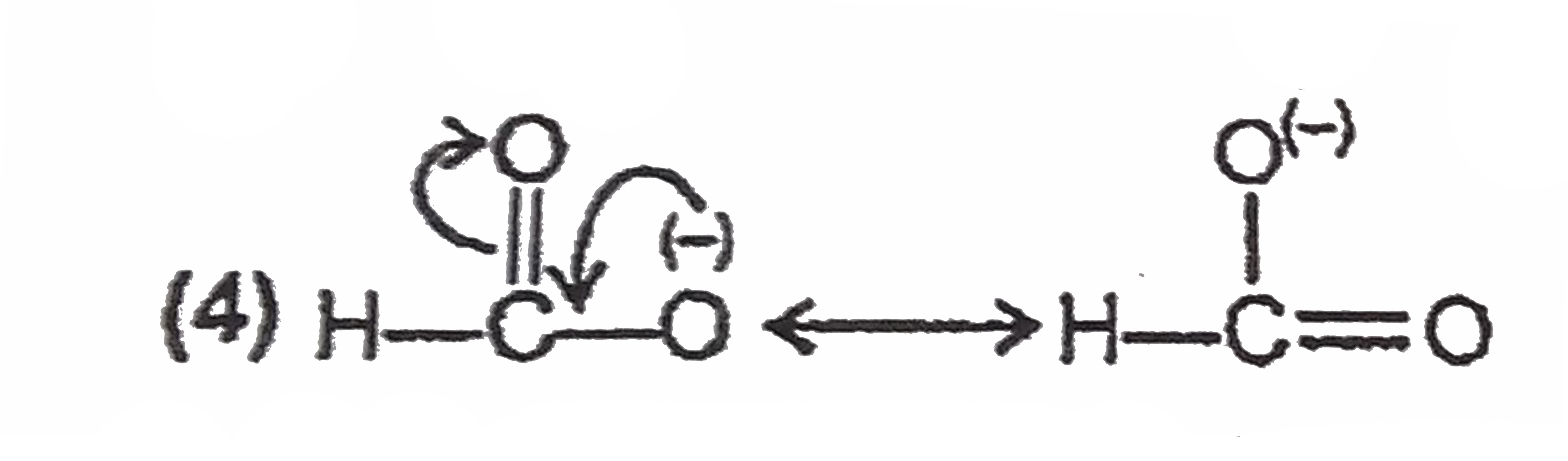
In HCOO−, the two carbon oxygen bonds are of equal length because the anion HCOO− has two equally stable resonating structures.
Similar Questions
In "HCOO"^(-) , the two carbon-oxygen bonds are found to be of equal length. What is the reason for this ?
View SolutionIn the anion RCOO^(-) , the two carbon -oxygen bonds are found to be of equal length. What is the reason for it?
View SolutionDebt HCOO^(-) The lengths of the two carbon-oxygen bonds are similar, this is because
View SolutionIn the HCOO− the two carbon-oxygen bonds are found to be of equal length. What is the reason for it ?
View SolutionIn the anion HCOO^(-) the two carbon-oxygen bonds are found to be of equal length . What is the reason for it ?
View SolutionIn HCOO- ion, the two carbon-oxygen bonds are found to be equal length, what is the reason for it?
View SolutionDebt HCOO^(-) In, two carbon – oxygen bonds of similar length are found. What is the reason for this?
View SolutionIn the anion HCOO^- , the carbon-oxygen bonds are found to be of equal length. This is due to :
View Solution
